All Features

Alex de Vigan
D uring the past decade, manufacturers have wired their plants with sensors, robots, and software. Yet many “AI-driven” systems still miss the mark. They analyze numbers but fail to understand the physical reality behind them: the parts, spaces, and movements that make up production itself.
“…

Harish Jose
In this article I want to explore an observation on how we make distinctions and what this reveals about the structure of our thinking. I’m inspired by the ideas in George Spencer-Brown’s Laws of Form and broader themes in cybernetics about how observers construct meaning.
The starting point is…

Lexi Sharkov
We’d be willing to bet your key collaborators aren’t all in the same building. Your team members, contract partners, clients, and suppliers are likely scattered across the globe. That makes collecting physical, “wet ink” signatures nearly impossible and turns digital approvals into a daily…
James J. Kline
The use of artificial intelligence (AI) models, specifically generative AI, is growing. This has raised concerns about the effects on jobs in various professions. The quality profession is among them.
Like it or not, the quality profession has been disrupted. This occurred before AI became widely…

Cassondra Blasioli
Growing up in Pittsfield, Massachusetts, I witnessed firsthand the heartbeat of American manufacturing. I remember the hum of machines, the rhythm of assembly lines, and the pride of workers crafting products that powered industries across the nation. I can still smell the oil and hear the machines…

Andrew Iams
I grew up outside Pittsburgh, widely known as “Steel City.” Although the city is no longer the center of steel or heavy manufacturing in America, its past remains a proud part of its identity.
Like many Pittsburghers, my family’s story is tied to this industrial legacy. My relatives immigrated…

MasterControl Inc.
Ninety days to implementation vs. 12 to 18 months with traditional systems: That’s not just an incremental improvement—it’s a complete reimagining of what’s possible in life sciences quality management.
In the highly regulated life sciences industry, quality management system (QMS) implementations…

Dolf van der Haven
When businesses talk about customer experience, the conversation almost always focuses on the end user. That’s understandable, but dangerously narrow. In modern service ecosystems, particularly those governed by service integration and management (SIAM), the customer experience depends just as much…
Harikrishna Kundariya
AI has amazing capabilities, and it’s one of the best technologies for the future. It’s helping to change the world and bringing productivity enhancements across industries with its exceptional use cases. Quality assurance isn’t left out, either. AI is highly useful in any product development…

Robin F. Goldsmith
Many organizations have decided to automate their quality management system (QMS) or upgrade their currently automated QMS. Quality management tends to involve significant numbers of documents, which automated systems are especially efficient at creating, accessing, tracking, updating, and…

George Schuetz
Digital calipers are one of the most common hand tools used on the shop floor. In a manufacturing plant, under a quality control system, these tools must be checked and calibrated regularly.
Past articles have discussed the pros and cons of doing gauge calibrations internally or by an external…

Knowledge at Wharton
The case for in-office work has never been stronger. This may seem surprising, given that many businesses have operated remotely or in hybrid models for more than five years. The initial excitement about remote work stemmed largely from its immediate conveniences and the individual flexibility it…

Lexi Sharkov
When an issue arises, it’s important to take quick action. Whether that means launching a software patch, pulling a batch, or halting the use of a reagent, it’s critical to tackle the immediate problem.
But just as critical as “How do we fix this?” is “How do we make sure this doesn’t happen again…

Knowledge at Wharton
My first day as a consultant coincided with the annual women’s summit, and so the other female new hires and I were invited to join the event. The firm had brought in a “gender empowerment” speaker who mostly seemed focused on addressing the mistakes women made in the workplace.
She went around…

David Mihal
Quality Digest was recently fortunate enough to get more information on Geomagic Design X for reverse engineering from David Mihal, global commercial director of the Geomagic software product line within Hexagon’s Manufacturing Intelligence division. The newly available software converts data from…
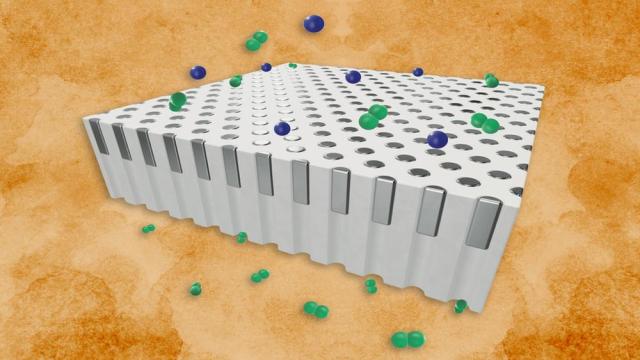
Jennifer Chu
Palladium is a key to jumpstarting a hydrogen-based energy economy. The silvery metal acts as a natural gatekeeper against every gas except hydrogen, which it readily allows through. For its exceptional selectivity, palladium is considered one of the most effective materials for filtering gas…

Charleen Newland
Many organizations list continuous improvement (CI) as a priority, but it often fails to take root in day-to-day work. It appears as a workshop or a one-off initiative, then fades without lasting change. If the goal is long-term performance and sustainable growth, CI can’t be incidental or optional…

Silke von Gemmingen
Powder bed-based laser melting of metals (PBF-LB/M) is a key technology in additive manufacturing that makes it possible to produce highly complex and high-performance metal components with customized material and functional properties. Used in numerous industries from aerospace and medical…

Dennis Wylie
You’ve probably had the experience of visiting a contemporary factory floor and being amazed by all the incredible robots, sensors, and machines working like a finely choreographed dance. It’s quite remarkable—until there’s a malfunction. And that’s something which has frustrated quality engineers…

NIST
In the wake of Covid-19 and widespread wildfires, demand skyrocketed for air cleaners, machines that could remove potentially harmful particles from the air in a home. Manufacturers responded by producing a wider variety of air cleaner devices designed for single rooms.
The main purpose of an air…