All Features

Anthony Vianna
There’s a lot of talk about automation these days, not just in manufacturing circles but also the news in general. As the demands of modern manufacturing grow more complex, and manufacturing industries continue their digital transformation—with automation playing an ever-expanding role—where does…

Quality Digest, Jason Chester
Today, manufacturing is largely shaped by supply chain volatility, complex labor dynamics, and—like most global industries—the rise of AI. Adopting AI technologies on the shop floor can help manufacturers minimize operational costs, mitigate risk, and optimize processes, which drives efficiency and…
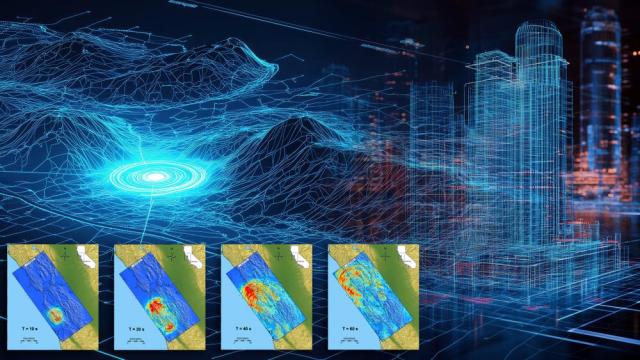
Oak Ridge National Laboratory
Simulations still can’t predict precisely when an earthquake will happen. Still, with the incredible processing power of modern exascale supercomputers, they can now predict how they will happen and how much damage they will likely cause.
Imagine a colossal earthquake strikes the California coast…

Walter Nowocin
Software selection, implementation, and ongoing maintenance are critical stages in the life cycle of biomedical software systems such as asset and calibration management platforms. Yet few industry resources provide detailed, practical guidance for managing these processes effectively.
One notable…
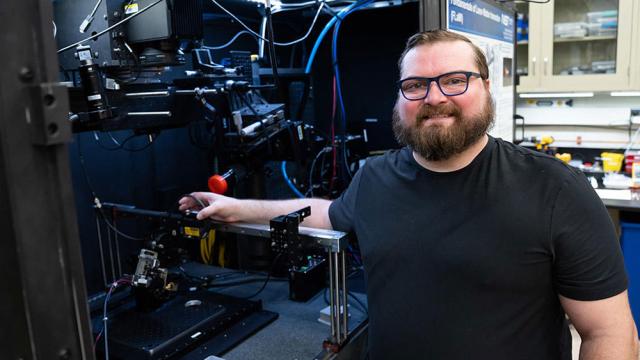
NIST
Even if you’ve never heard of “light caustics,” you’ve probably seen them. They’re the ethereal patterns of light that coat the bottoms of swimming pools and break up the shadows of glass. Anywhere light is bent or reflected by a curved surface, caustics can appear.
This trick of the light is…

Annie Wilson, Professor Ryan Hamilton
Nano Tools for Leaders, a collaboration between Wharton Executive Education and Wharton’s Center for Leadership and Change Management, are fast, effective tools that you can learn and start using in less than 15 minutes, with the potential to significantly affect your success and the engagement and…

Frank King
At Ramirez & Co., a midsize business with decades of wins, leadership thought its biggest challenges were competitors, technology, and the market. Close, but no cigar. The real problem was stress, the silent drain that doesn’t show up on a Gantt chart but still wrecks your timeline.
Deadlines…

Enterprise Innovation Institute at Georgia Tech
A new study explains how tiny water bugs use fan-like propellers to zip across streams at speeds up to 120 body lengths per second. The researchers then created a similar fan structure and used it to propel and maneuver an insect-sized robot.
“Scientists thought the bugs used their muscles to…

Gleb Tsipursky
Imagine a bustling conference room where employees aren’t just listening to lectures but actively experimenting with cutting-edge tools, tackling real-world challenges and discovering new ways to revolutionize their workflows. That’s the transformative power of workshops focused on generative AI (…

Mike Figliuolo
It’s hard to balance all the demands that are placed upon you as a leader. Many of us default to dysfunctional ways of spending our time and energy. If you know what the common mistakes are and take a more deliberate approach to investing your time and energy, you’ll get better results from your…

Judy Fainor
What if your quality system could detect and initiate corrective actions for equipment deviations before they affect product quality?
It’s a compelling vision—and one that’s becoming increasingly achievable through AI-enabled automation. But let’s be clear: We’re not there yet. What we do have is…

Cornelia C. Walther
Sarah, a marketing director at a Fortune 500 company, recently celebrated her team’s 40% productivity increase after implementing AI-powered content generation tools. Her seasoned copywriters now produce campaigns in hours rather than days, while AI handles routine social media posts and email…

Mike Regan
In July 2024, CrowdStrike rolled out a software update that crashed more than 8 million Windows systems worldwide. The faulty release disrupted hospitals, grounded flights, halted banking operations, and affected government services. Comparable to a major cyberattack, the incident caused more than…

Kate Zabriskie
Most of us have been there. A deadline whooshes by, a teammate consistently shows up late to meetings, or someone just isn’t pulling their weight. And what do we often do? We avoid the conversation, hoping the problem will magically resolve itself. Spoiler: It never does.
Dodging these…

Bruce Hamilton
In my Labor Day article, “Celebrating Our Frontline Scapegoats,” I observed that of the seven wastes, the one most people recognize is defects. This is understandable: Workers are often blamed for defect-causing situations over which they have little or no control. This article continues that Labor…

Kateryna Sergieieva
Organizations today face a problem that’s both simple and enormous: They operate in a world that moves faster than the systems used to track it. Shipping bottlenecks appear overnight, suppliers run into trouble without warning, and crops or energy grids can shift dramatically in just a few days. In…

William A. Levinson
A vital concept from the chemical process industry, management of change (MOC) relates primarily to safety. It means that whenever we change a factor in a cause-and-effect diagram (e.g., machine, material, manpower, method, measurement, environment, or any other factor), we create risks of…

Akhilesh Gulati
Organizations often face a familiar dilemma: It’s not a shortage of good ideas, but a struggle to decide which one to pursue first. During project prioritization meetings, leaders are likely to present a wide range of perspectives. The finance team pushes for hard savings, while operations advocate…

Cindy Mielke
Over the years, there have been many developments in systems and technologies that have helped industries redefine how businesses are structured. These changes, especially in sectors like manufacturing and distribution, have led to new roles and responsibilities for employees.
However, often with…

Carl Lewis
Manufacturers are making more types of products than ever before. According to Deloitte’s Consumer Products Industry Outlook, 95% of consumer product executives report that launching new products is a top priority this year, and 67% are allocating more resources to developing truly novel offerings…
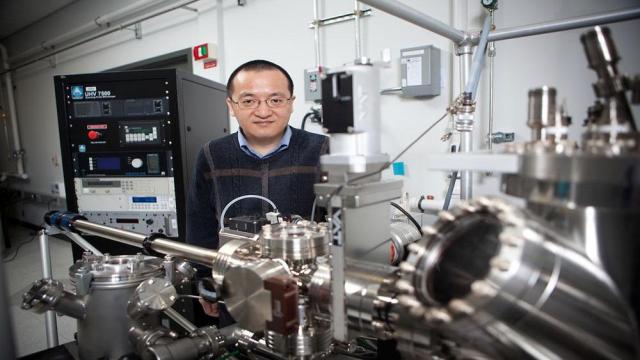
Brookhaven National Laboratory
Most metals found in nature are actually in their oxide forms. To extract those metals to use in critical applications—ranging from infrastructure such as bridges and buildings to advanced technologies like airplanes, semiconductors, or even quantum materials—those oxides must be reduced with gases…
Donald J. Wheeler
Everybody wants to have good measurements. To this end, many recommend a regular schedule of recalibration. While this sounds reasonable, it can actually degrade the quality of the measurements.
The key to getting the most out of a measurement process is to know when to recalibrate and when to…

Jake Walton
Imagine you’re a student trying to pass a challenging class, one where the entire grade rests on the big test at the end of the semester. Fortunately, the professor handed out a syllabus that outlines exactly what will be on that final exam. Better still, you can also find a posted list of common…

Global Shop Solutions
Cutting costs is nothing new in manufacturing. What’s new is having to do it while juggling labor shortages, supplier delays, and tighter customer demands. Lean principles such as reducing waste and optimizing workflows still matter, but they’re no longer enough on their own. Staying competitive…