All Features

Bryan Christiansen
An important part of production is to carefully monitor and control temperature, speed, volume, weight, or mass. To ensure these measurements are always accurate, manufacturers need to calibrate their equipment and instruments regularly.
Devising a proper equipment calibration schedule can be a…

Del Williams
With the threat of contamination from harmful pathogens such as salmonella, listeria, and e. coli a continual concern, food processors are seeking to protect not only the public but also their companies’ bottom lines from the massive costs, reputational damage, and greater regulatory scrutiny…
Anton Ovchinnikov
In the age of mass production, the demand for customization is increasing. Customers prefer products catered to their individual needs and preferences over standard items—albeit at a cost.
Fortunately, recent advances in information technology, logistics, and advanced manufacturing processes such…

Christopher Dancy
Despite the important and ever-increasing role of artificial intelligence in many parts of modern society, there is very little policy or regulation governing the development and use of AI systems in the United States. Tech companies have largely been left to regulate themselves in this arena,…

Jake Mazulewicz
A technician spills a toxic chemical. She isn’t injured but easily could have been. The hazmat cleanup costs more than $10,000 and shuts down a critical building for a week.
An electrical engineer flips the wrong switch in a substation control room. He isn’t injured. But within seconds, a $50,000…
NIST
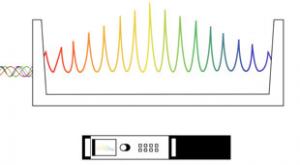
An improvement to a Nobel Prize-winning technology called a frequency comb enables it to measure light pulse arrival times with greater sensitivity than previously possible—potentially improving measurements of distance along with applications such as precision timing and atmospheric sensing.
The…

Bryan Christiansen
Assets are resources owned and used by a company to generate a positive economic benefit. Assets can be physical items, like equipment or furniture, or they can be intangibles like software, patents, or documents.
As a business owner, it’s important to know which assets you own, their location,…

Leeza Garber, Allison Jegla
In late spring 2022, the U.S. Securities and Exchange Commission (SEC) charged an elite investment adviser for “misstatements and omissions” about environmental, social, and governance (ESG) considerations related to its managed mutual funds. This same financial firm has also faced myriad…

Gleb Tsipursky
BlackRock CEO Larry Fink claimed in a recent interview with Fox that “we have to get our employees back in the office.” According to him, doing so would result in “rising productivity that will offset some of the inflationary pressures.”
Fink didn’t provide any data in the form of statistics,…

Jeff Dewar
This is the fourth installment of a five-part series.
As detailed in our third installment, ASQE is a new legal entity connected to the ASQ we all know and love. It’s a trade organization to which organizations, rather than individuals, can belong. Current membership is about 180 organizations,…

Quy Huy
In September 2022, Boeing agreed to pay $200 million for charges that it misled investors about two crashes of its 737 Max aircraft that killed 346 people. The penalty imposed by the U.S. Securities and Exchange Commission is small change compared to the $2.5 billion shelled out by the plane maker…

Jon Speer
Medical device product development and risk management are often treated as entirely separate processes. Sure, there is usually acknowledgement and understanding that these two processes are related. But it is important to realize that product development and risk management share more than that.…

Tom Taormina
It’s a conundrum that faces everyone who operates a manufacturing or service business: Most are unaware of the dire consequences of a defect reaching a customer until a process server hands them a lawsuit. By then it’s too late. Regardless of the outcome, the people and businesses will be…

Pat Toth
In an earlier article in this series, “Cybersecurity and Industry 4.0: What You Need to Know,” we discussed the four aspects of Industry 4.0: cyber-physical systems (CPS)/cobots, internet of things (IoT), cloud manufacturing, and automation, as well as how they are interconnected. Strong…

Patrick Hardy
Responding to disasters is one of the most important activities that employees can be asked to grapple with. From natural disasters like hurricanes and earthquakes to technological situations such as power outages, chemical spills, and transportation accidents, as well as security emergencies like…

Dirk Dusharme
Every company wants to succeed, but not all can say they meet the current requirements to do that. More than a focus on capital, business plans, or staff, a successful business in 2022 must operate digitally. Yet for the 45 percent of small and medium-sized businesses (SMBs) that still rely on…

David Isaacson
In 1982, when asked about the state of the company’s Xenix operating system, a Microsoft engineer reportedly called it “vaporware“ to indicate that the operating system had really not yet materialized. Unfortunately, the term stuck for this and many other premature software launches.
It’s not only…

John Logan
Labor Day 2022 came smack-bang in the middle of what is increasingly looking like a pivotal year in the history of American unions.
The summer saw a steady stream of workforce mobilizations. Employees at Trader Joe’s locations in Massachusetts and Minneapolis both voted to unionize. Meanwhile,…

Gleb Tsipursky
Recently, JPMorgan CEO Jamie Dimon claimed that returning to the office will help improve diversity. If he’s right, that’s an important argument for office-centric work. After all, extensive research shows that improving diversity boosts both decision-making and financial performance.
Yet does…

Abdul Salam
Water is the most essential resource for life, for both humans and the crops we consume. Around the world, agriculture accounts for 70 percent of all freshwater use.
I study computers and information technology in the Purdue Polytechnic Institute, and direct Purdue’s Environmental Networking…

Gene Kaschak
Many manufacturers that adopted lean principles by applying a “just-in-time” (JIT) mindset to inventory of materials and parts have been burned, sometimes badly, by cascading supply chain disruptions. Broken links in the supply chain have created havoc, especially for smaller manufacturers.
Some…
Dwayne Duncum
The workplace has changed forever, having gone through a revolution similar to the Industrial Revolution. Our workplaces are diverse, complex, and frequently changing. If we take any lesson from the Covid pandemic, it’s that the way we work, where we work, and how we work have fundamentally shifted…

Etienne Nichols
I know what you’re thinking. You’ve got a medical device prototype that the FDA has categorized as Class I. You’re ready to push forward to manufacturing or marketing the device, since there are no formal requirements for design controls. “So why would I waste time on design controls?”
The fact is…

Sam Hunter, Gina Ligon
There’s a well-known aphorism that generals are always fighting the last war. It’s a natural human tendency to focus on the kinds of threats you’re used to while playing down the likelihood or importance of some new sort of attack.
Of course, novel threats can crop up anytime and anywhere. An…

Bryan Christiansen
CNC (for computer numerical control) machines have made manufacturing easier, faster, and more precise. Supported by the development of IoT technology, the CNC machine market is set to experience significant growth. With that in mind, this seems like a great time to discuss the intricacies of CNC…