All Features
NIST
Static force, such as the weight of a person standing motionless on a bathroom scale or the force that an office full of equipment exerts on a high-rise floor, can be easily determined using scales, balances, load cells, and the like because static force doesn’t change over time. It’s…
Etienne Nichols
On Feb. 23, 2022, the U.S. Food and Drug Administration (FDA) released its proposed rule for the new Quality Management System Regulation (QMSR). The proposed QMSR will be the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s Quality System Regulation (QSR…

Michael Jarrett
History is filled with tales of courageous and decisive heroes. Individuals like Julius Caesar and Winston Churchill, for example, have led from the front to guide people through adversity and achieve ultimate success. This myth building is especially prominent in business, with stories of…

Ian Wright
It’s been a long and arduous road, but you’re almost ready for that first production run. You made it through supplier selection, your designs and production processes have been finalized, preproduction is finished, and now there’s just one more hurdle to clear: first article inspection (FAI).…

Stephanie Ojeda
Mistakes around standard operating procedure (SOP) management are widespread and costly, especially given the pace of change in manufacturing today.
Consider, for example, an electronics manufacturer that introduces a new product model with updated features and components. This new model requires…

Etienne Nichols
Your company probably has an internal process for a large purchase like an eQMS. In midsize-to-large medtech companies, you’ll likely find this process in the finance department, or perhaps in a dedicated purchasing department operating under finance’s umbrella.
1. Start by learning about your…

Kari Miller
Since 2010, citations for insufficient corrective action and preventive action (CAPA) procedures have been at the top of the list of the most common issues within the U.S. Food and Drug Administration (FDA) inspections, particularly for the medical device industry. Issues can occur while…

Etienne Nichols
Amedical device company is expected to deliver innovative, life-changing devices while ensuring compliance and achieving true quality. This task bears loads of responsibility—all of which must be kept and documented within your quality management system (QMS).
A QMS contains everything that…

Hank C. Andersen
Not many years ago, there was a CEO so exceedingly fond of finding the right strategy that he spent all of his money on consultants to tell him what the strategy should be. One day there came two consultants, and they said they could craft the most magnificent strategy imaginable. Not only would it…

Megan Wallin-Kerth
Business owners and employees alike have long debated over how best to achieve quality standards and what those standards ought to be. However, as much as linear thinking may help when measuring degrees of improvement, increases in profit, or low turnover rates, it can’t tell you that your company…

Aaron Smith
A successful company can’t run without happy and motivated employees. One way you can achieve that is by improving your employees’ uptime. Uptime refers to your employees’ freedom to pursue personal and occupational growth without the burden of preventable injuries. Here is everything you need to…

Stephanie Ojeda
Design controls are a frequent citation in 483 observations and warning letters from the U.S. Food and Drug Administration (FDA). In fact, the agency has noted a large proportion of past recalls that could have been prevented with design controls.
FDA guidance also makes an explicit link between…

ISO
Net zero is our strongest tool yet against the climate crisis. The transition to net-zero emissions presents a compelling solution that offers not only environmental benefits but also economic, social, and health advantages. Failing to act swiftly and decisively risks catastrophic climate change,…
Sébastien Breteau
Supply chain quality control is a demanding job. Ensuring that products meet specific standards and expectations for safety and customer satisfaction by monitoring and managing the entire supply chain, from raw materials to finished products, must be accomplished consistently and reliably.
At any…

Etienne Nichols
The goal of your MedTech company’s supplier management process should be to ensure a consistent supply of high-quality parts and components that conform to your specifications.
But achieving that goal is easier said than done, and it depends heavily on whether you take a risk-based approach to…

Ian Wright
‘I have a cellphone that doesn’t behave like a phone: It behaves like a computer that makes calls. Computers are becoming an integral part of daily life. And if people don’t start designing them to be more user-friendly, then an even larger part of the population is going to be left out of even…
Michael King
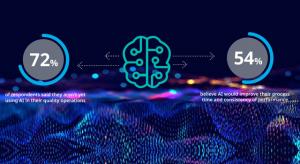
Medical companies work in an environment of ever-increasing challenge and complexity. Global regulations continue to evolve with advancements in technology and variations in requirements from country to country. This brings unavoidable technical complexity to daily tasks of quality and regulatory…

Melissa Phillips
The levels of contaminants in our food supply are, generally, decreasing. That’s the good news.
But we still need to measure those contaminants and make sure our food is safe. And measuring tiny things (and big things) is what we do best here at NIST.
In our food safety program, we’re studying all…

Jessie MacAlpine
Despite rigorous vehicle safety testing, current evaluations fail to adequately protect women in the case of an accident. In 2021, a study published by the Insurance Institute of Highway Safety found that women have a mortality rate 20% to 28% higher than men during road accidents. Women are also…
Etienne Nichols
Medical device companies must have established risk management processes that comply with ISO 14971. It doesn’t matter whether you’re developing medical devices in the U.S., EU, Canada, or elsewhere. Every international regulatory agency you’ve ever heard of accepts ISO 14971.
ISO 14971 is a good…

Jake Mazulewicz
Wildland firefighters. Air traffic controllers. Flight deck crews of aircraft carriers. Operators of nuclear power plants and the national bulk electric grid. These are among the safest and most reliable work teams in the world. And they don’t try to eliminate all errors and surprises.
Decades of…

Harish Jose
I’m looking at a topic in statistics. I’ve had a lot of feedback on one of my earlier posts on OC curves and how one can use them to generate a reliability/confidence statement based on sample size (n), and rejects (c). I provided an Excel spreadsheet that calculates the reliability/confidence…

Martin Cottam
It’s tempting to attribute the increased profile now given to occupational health and safety (OH&S) to the Covid-19 pandemic. But while in many organizations the pandemic shone a spotlight on OH&S management, there are other issues that will keep OH&S at the fore throughout the next…

Ken Chapman, Tony Orlowski
‘Turning to the men around him, Dodge shouted, ‘Up this way!’ but the men ignored him. Dodge later stated that someone responded, ‘To hell with that, I’m getting out of here.’ The team raced past Dodge up the slope toward the ridge. Four men reached the crest, but only two, Bob Sallee and Walter…

Bryan Christiansen
With so many assets and projects to think about, facilities management is a huge and complex field. It’s easy to lose focus.
Luckily, there’s a simple and easy fix for this problem—facility management KPIs (key performance indicators). Outlining and tracking the most important ones will help you…