All Features

Etienne Nichols
Good supplier management is one of the most important methods of building a safe and effective medical device. A single device may be made up of dozens of parts and components coming from several different suppliers, and many medical device companies outsource the manufacturing of their device to a…

Akhilesh Gulati
Complacency won’t show up on a control chart. But its damage is real. Can AI and systems thinking help us detect it and respond before trust is lost?
As customer expectations evolve, one question remains: Are customers still at the core of your company’s operations?
Back in 1999, a simple but…

Sabine Terrasi
The precise recording of passenger numbers is essential for transport companies—it helps optimize timetables, make better use of capacities, and organize local public transport more efficiently.
A modern solution for this is camera-based passenger-counting. Intelligent image processing systems…

ISO
The digital revolution has transformed healthcare along with virtually every other industry. From telemedicine to digital health data, providers now have access to innovative solutions that have the potential to make healthcare more accessible and effective for all.
In some instances, this is done…

John Tschohl
Why do customers patronize one company over another? Many of you might say that the quality and price of the products or services are key factors. But while those things might play into a purchasing decision, they aren’t the most important consideration.
So, what is? Customer service. How you and…

Knowledge at Wharton
Many countries face the reality of demographic aging: Fertility is plummeting and people are living longer. This raises critical challenges for the labor market, healthcare, and long-term care markets, as well as retirement systems and financial planning. A Wharton symposium on the implications of…

Amy Knue
Health systems across the country are unknowingly paying multiple times for the same medical equipment—once to own it, and again to rent it. The issue isn’t always an increase in clinical demand; it’s often availability and visibility to medical device inventory. The cost of these unnecessary…

Etienne Nichols
The corrective and preventive action (CAPA) process is one of the most important elements within a medtech company’s quality management system (QMS). The goal of the CAPA system is to identify, address, and prevent systemic issues that could compromise product safety, regulatory compliance, and the…

Mike Figliuolo
Most days we walk through life unaware of the conversations occurring around us. And then there are those times you overhear a conversation that stops you dead in your tracks. You have to hit rewind in your brain and ask, “Did they actually just say that?”
Ever have one of those moments? Clearly,…

Stephanie Ojeda
Many companies are still clinging to paper-based and unconnected electronic processes, despite the clear disadvantages. Without modern tools like QMS software, these organizations risk compromising product quality, falling behind in compliance, and ultimately losing competitive ground.
In contrast…

NIST
Using an electron beam to image the tiniest of defects and patterns on microchips, the scanning electron microscope (SEM) has long been a mainstay of the semiconductor industry. But as the industry continues to miniaturize chip components—essential for computers, implantable drug dispensers,…

Prasant Prusty, Arundhathy Shabu
A global food safety and quality certification, BRCGS (British Retail Consortium Global Standards) initially focused on food safety but now comprises various sectors such as packaging, consumer products, and retail. It aims to ensure that businesses maintain high standards of safety and quality…
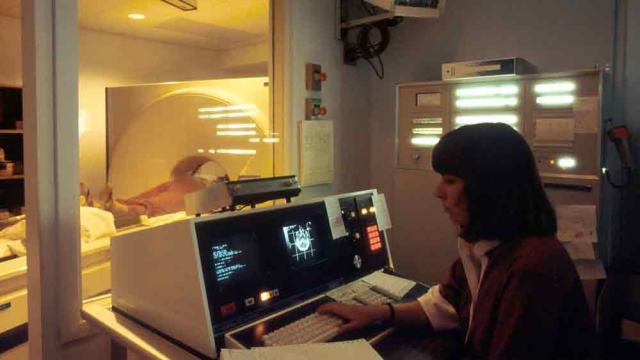
Etienne Nichols
As part of its effort to address the changing landscape around artificial intelligence (AI) in medical devices, the U.S. Food and Drug Administration (FDA) has recently released two new guidance documents on artificial intelligence-enabled device software functions (AI-DSF): • “AI-enabled device…

Cornelia C. Walther
On April 8, 2025, a driverless Zoox robotaxi misjudged an approaching vehicle, braked too late, and sideswiped it at 43 mph on the Las Vegas Strip.
One month later, the Amazon subsidiary issued a software recall on 270 autonomous vehicles and suspended operations while regulators investigated the…

Heidi Drafall
Anyone who has cracked their smartphone screen or had a rapid oil change knows that sometimes the OEM isn’t the most affordable or convenient service option. Consumer flexibility, paired with lower-cost, high-quality options, is logical, whether it’s in the consumer market or in healthcare.
The…
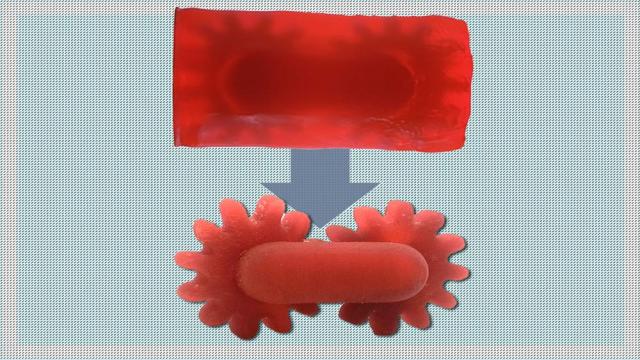
Jennifer Chu
Hearing aids, mouth guards, dental implants, and other highly tailored structures are often products of 3D printing. These structures are typically made via vat photopolymerization—a form of 3D printing that uses patterns of light to shape and solidify a resin, one layer at a time.
The process…

William A. Levinson
According to the U.S. News & World Report article “FDA Warns Sanofi of Manufacturing Irregularities at Key Facility” (Jan. 23, 2025), the pharmaceutical company Sanofi received a U.S. Food and Drug Administration warning letter “stating that FDA inspectors found irregularities with the facility…

Creaform
The Marshall Advanced Manufacturing Center (MAMC) is a leading-edge resource facility dedicated to driving innovation and advancing manufacturing technologies.
Operating from West Virginia facilities in Huntington, South Charleston, and Point Pleasant, the MAMC is at the heart of groundbreaking…

Kate Zabriskie
You know that friend who’s always there when you’re in need? The one who shows up on time, follows through on promises, and genuinely cares about what’s going on in your life? That’s exactly what your business needs to be for your customers.
Sure, those splashy marketing campaigns and point-…

Sunderesh Heragu
According to the U.S. Census Bureau and the U.S. Bureau of Economic Analysis, trade with our three largest partners—Canada, China, and Mexico—accounted for more than $1.32 trillion in imports and $0.82 trillion in exports in calendar year 2024. This represented 40% of the total trade between the…
Matt McFarlane
One of the key findings in Greenlight Guru’s 2025 Medical Device Industry Report was that economic uncertainty is playing a large role in the decisions medical device companies make this year.
The report surveyed more than 500 medical device professionals across quality, regulatory, product…

Jennifer Chu
For a robot, the real world is a lot to take in. Making sense of every data point in a scene can take a huge amount of computational effort and time. Using that information to then decide how to best help a human is an even thornier exercise.
Now, MIT roboticists have a way to cut through the data…

Bruce Hamilton
A few months ago I visited a potential customer, a high-tech startup, which like many Boston-area tech companies is developing astounding products that would have been considered science fiction only 10 years ago. The parking lot was half full at 8 a.m., but the entrance was locked to visitors, and…

Stephanie Ojeda
Every day, quality leaders face a variety of production and process issues. Although some problems are easy to fix, others require deeper investigation, such as using a 5 Whys analysis or fishbone diagram. But then there are the stubborn, recurring issues that can lead to quality issues, increased…

Akhilesh Gulati
When we step into a complex organization—whether in manufacturing, healthcare, or finance—we often find ourselves navigating a sea of competing truths. Everyone seems certain they see the problem clearly. Yet somehow, solving it feels harder than it should.
Why?
Often, it’s not the facts that…