All Features
Maartje van Krieken
Your market is shifting, your competitor just pulled ahead, and the one person who could execute the next move has resigned. You can’t get more data fast enough, yet the window to act is closing. In today’s volatile, uncertain, complex, and ambiguous (VUCA) world, this is the moment that defines…

Global Shop Solutions
Small to midsize manufacturers are facing mounting pressure from unpredictable supply chain disruptions. From fluctuating customer demand to reshoring operations and diversifying suppliers, maintaining efficiency and protecting cash flow have never been more critical.
Global instability, shifting…

Walter Nowocin
Software selection, implementation, and ongoing maintenance are critical stages in the life cycle of biomedical software systems such as asset and calibration management platforms. Yet few industry resources provide detailed, practical guidance for managing these processes effectively.
One notable…

Annie Wilson, Professor Ryan Hamilton
Nano Tools for Leaders, a collaboration between Wharton Executive Education and Wharton’s Center for Leadership and Change Management, are fast, effective tools that you can learn and start using in less than 15 minutes, with the potential to significantly affect your success and the engagement and…

Frank King
At Ramirez & Co., a midsize business with decades of wins, leadership thought its biggest challenges were competitors, technology, and the market. Close, but no cigar. The real problem was stress, the silent drain that doesn’t show up on a Gantt chart but still wrecks your timeline.
Deadlines…

Judy Fainor
What if your quality system could detect and initiate corrective actions for equipment deviations before they affect product quality?
It’s a compelling vision—and one that’s becoming increasingly achievable through AI-enabled automation. But let’s be clear: We’re not there yet. What we do have is…

Mike Regan
In July 2024, CrowdStrike rolled out a software update that crashed more than 8 million Windows systems worldwide. The faulty release disrupted hospitals, grounded flights, halted banking operations, and affected government services. Comparable to a major cyberattack, the incident caused more than…

William A. Levinson
A vital concept from the chemical process industry, management of change (MOC) relates primarily to safety. It means that whenever we change a factor in a cause-and-effect diagram (e.g., machine, material, manpower, method, measurement, environment, or any other factor), we create risks of…

Carl Lewis
Manufacturers are making more types of products than ever before. According to Deloitte’s Consumer Products Industry Outlook, 95% of consumer product executives report that launching new products is a top priority this year, and 67% are allocating more resources to developing truly novel offerings…

Jake Walton
Imagine you’re a student trying to pass a challenging class, one where the entire grade rests on the big test at the end of the semester. Fortunately, the professor handed out a syllabus that outlines exactly what will be on that final exam. Better still, you can also find a posted list of common…

Sachin Waikar
Take two aspirin and call me in the morning: If only prescribing medications were as simple as that.
In reality, the prescription process involves many players and steps. Details must be accurately spelled out, interpreted, and double-checked to ensure patients get the correct drug and dosage.
“…

Creaform
C hallenges abound for today’s manufacturers. Labor shortages and rising labor costs require innovative solutions to maintain productivity with fewer staff. Inflation continues to exert pressure on raw material costs, squeezing margins. Manufacturers are also racing against tight production…

Susan Robertson
Productivity looks good on paper. It’s measurable, visible, and in many organizations, it’s worshipped. But here’s the problem: Productivity isn’t the same as progress.
Many cultures confuse motion with momentum. Leaders celebrate packed calendars, rapid responses, and efficiency hacks, but often…

Kamran Sayrafian
A few years ago, I heard on the news that many people were being hospitalized with a condition of excess fluid in the lungs, called pulmonary edema. It’s common in elderly patients. Pulmonary edema is dangerous and can lead to breathing difficulties and lung failure. Because it has the potential to…

Paul Hanaphy
The dental industry is seeing a surge in 3D printing, with the technology enabling a growing number of dentists to rapidly create custom implants in clinics around the world.
When it comes to customizing implants like dental crowns, bridges, guides, and aligners, 3D printing is faster, more…
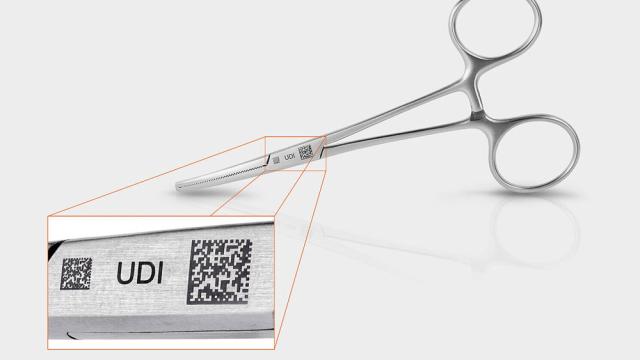
FOBA
In medical technology, safety is paramount. Every step in the manufacturing process must meet the highest standards, from patient protection to regulatory compliance. Because direct part marking plays a key role in ensuring traceability and unique device identification (UDI) conformity, it’s…

NIST
A rapidly growing category of drugs called protein-based biotherapeutics can be used to treat cancers and genetic and autoimmune disorders. These drugs, which usually take the form of large protein molecules, are manufactured by growing living cells that are genetically engineered to produce the…

Creaform
The Bott Group designs vehicle and operating equipment as well as workplace systems. From its headquarters in Gaildorf, Germany, and two production sites in Bude, England, and Tárnazsadány, Hungary, Bott Group designs and manufactures work environments for mobile and stationary manual work…

William A. Levinson
I recently needed to have a hot water expansion tank installed in my house. The first plumber who came to mind is widely advertised on local radio. The company’s online reviews suggest that they do good work, but one added that they are expensive—and it’s probably because they have radio ads…
Nimax
Pharmaceutical serialization practices are on the rise and have progressively become a worldwide standard as a result of stringent regulations in various of markets, including the United States, European Union, China, and Argentina. Recent estimations found that by 2022 serialization practices had…

Ariana Tantillo
A new security screener that people can simply walk past may soon be coming to an airport near you. Last year, U.S. airports nationwide began adopting HEXWAVE, a commercialized system based on microwave imaging technology developed at MIT Lincoln Laboratory. HEXWAVE addresses a new Transportation…

Oak Ridge National Laboratory
The U.S. Department of Energy’s Oak Ridge National Laboratory (ORNL) and JuggerBot 3D, an industrial 3D-printer equipment manufacturer, have launched their second research and development collaboration through the Manufacturing Demonstration Facility (MDF) Technical Collaboration Program.
The two…

Sam Schaffter
My grandfather was a carpenter, so growing up I often heard the adage, “Be sure to measure twice so you only have to cut once.” The saying is also attributed to tailors—you have to make sure your measurements are correct before you cut the fabric.
Little did I know that years later the saying…

Jennifer King
Although patient safety is paramount in healthcare settings, about 1 in 10 patients is harmed in healthcare, and more than 3 million deaths occur due to unsafe care, says the World Health Organization (WHO). The reality is hospitals and healthcare facilities face numerous challenges in managing…

Etienne Nichols
Good supplier management is one of the most important methods of building a safe and effective medical device. A single device may be made up of dozens of parts and components coming from several different suppliers, and many medical device companies outsource the manufacturing of their device to a…