All Features

Graham Freeman
Many industries have no clear boundary between safety and quality culture. In fact, they are often closely integrated. Quality failures and nonconformances that require rework have been correlated with increased accidents and recordable injury rates in manufacturing organizations. These injuries…

Wendy White
Starting a new facility in the food-processing industry is an enormous undertaking. There are thousands of things that must be accomplished, from hiring and training new staff to ordering and installing equipment. This scenario is a perfect example of “too much to do and not enough time to do it…

Chip Bell
Visioning beyond the customer is the responsibility of every person interested in a competitive advantage.
What do Bill Marriott, Ray Kroc, and Al Hopkins have in common?
No, they are not all people of wealth and fame. In fact, Hopkins is a small-town accountant and part-time preacher. They all…

Zac Cooper
The role of quality starts with product design and moves rapidly across the supply chain to the selling and buying experience, which includes the bidding process. When operating a formal continuous process improvement program, nearly all manufacturing engineers are tasked with some level of quality…

Dirk Dusharme
We interview Stanley Chao, author of Selling to China: A Guide for Small and Medium-Sized Businesses (iUniverse, 2018), about the impact of the current U.S.-China trade war. Does China really care, and where do U.S. multinationals go from here? Also, a quick look at Conformance Manager, a web-based…

Brian Strzempkowski, Shawn Pruchnicki
When Amelia Earhart took off in 1937 to fly around the world, people had been flying airplanes for only about 35 years. When she tried to fly across the Pacific, she—and the world—knew it was risky. She didn’t make it and was declared dead in January 1939.
In the 80 years since then, many other…

Wolfgang Ulaga
Offering free services may seem like a good way to keep customers happy, but how much money is your business leaving on the table? By redefining freebies as paid opportunities, B2B firms can generate new sources of income and secure long-term growth.
This not to say that companies should stop…

Stanley Chao
This past year has created havoc for Western companies purchasing raw materials, furniture, high-tech components, auto parts, and power tools from China. And the rocky relationship continues into 2019 as both countries continue to negotiate while kicking the can down the road toward a new March 1,…

Stephen Fankhauser, Matt Ebbatson
The world is running out of experienced pilots. Supply is not keeping up with the growing demand for air travel. In Australia, the effects are already starting to bite. Even flagship carrier Qantas is having problems. In recent months it has had to perform a very nimble tap dance to crew its vast…

Richard Harpster
On Oct. 13, 2018, the Automotive Industry Action Group (AIAG) sponsored a webinar on the status of the AIAG Core Tools Software (AIAG CTS). John Cachat, AIAG project manager for the AIAG CTS project, was the presenter for the webinar. The presentation provided information on why the AIAG was…

Dirk Dusharme
We tied up last year in a neat little bow, talking about how stories define ourselves and our work; waste is waste, no matter your political leanings; and putting numbers from the news in context.
“The Gift of Being Small” This article by Quality Digest’s Taran March wonderfully illustrates how we…

Bretta Kelly
Back in January 2009, I wrote an article for Quality Digest titled “ISO 9001 Documentation Is Like a Box of Chocolates.” Here we are, almost 10 years later, all of the ISO 9001 and related standards have been updated, yet companies still misunderstand what to document, how to document, or why to…
William A. Levinson
The U.S. government’s Fourth National Climate Assessment warns that climate change “creates new risks and exacerbates existing vulnerabilities in communities across the United States, presenting growing challenges to human health and safety, quality of life, and the rate of economic growth.” The…

NIST
A convocation of delegates representing 60 countries voted last month in Versailles, France, to implement the most significant change to the International System of Units (SI) in more than 130 years. For the first time, all measurement units will be defined by natural phenomena rather than by…

Wudan Yan, Knowable Magazine
Nearly every month, it seems, comes a new report. In March 2018, there was news of contaminated romaine lettuce, which eventually led to five deaths and sickened more than 200 people across the United States and Canada. In May 2018, about 100 people in California got sick after eating raw oysters…

NIST
The U.S. Department of Commerce announced that the 2018 Malcolm Baldrige National Quality Award will be given to two educational institutions, an organ donor group, a hospital, and a project management firm. A presidential-level honor, the award recognizes exemplary U.S. organizations and…

Dirk Dusharme
In this episode we look at lessons learned (or not) from GE, the difference between ISO and FDA “requirements,” and this year's Baldridge recipients.
“GE’s Lessons Won’t Determine Whether You Succeed or Fail”
Does the success or failure of GE’s CEO really matter that much when it comes to how most…

Mike Richman
This Thursday, for the first Thanksgiving in decades, I won’t be watching football, drinking beer, and stuffing myself into oblivion. Instead, I’ll be serving the homeless, giving clothes, and making myself useful.
Thanksgiving is always a time to give thanks—I mean, it’s right there in the holiday…
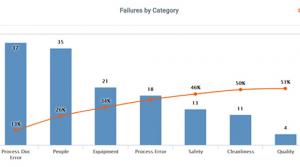
Paul Foster
Next to defining a problem accurately, root cause analysis is one of the most important elements of problem-solving in quality management. That’s because if you’re not aiming at the right target, you’ll never be able to eliminate the real problem that’s hurting quality.
So which type of root cause…

Amy Mahn
The NIST Cybersecurity Framework consists of standards, guidelines, and best practices to manage cybersecurity-related risk. The framework’s prioritized, flexible, and cost-effective approach helps to promote the protection and resilience of critical infrastructure and other sectors important to…
Ryan E. Day
BioBridge Global (BBG) is a parent organization for four subsidiary organizations, three of which are involved in production activities, and they’re all around regenerative medicine, including blood components, clinical laboratory testing, and cell and tissue therapies. Organizations in the life…

Taran March @ Quality Digest
These days, even regulatory agencies must innovate if they expect to keep pace with the speed of doing business. The U.S. Food and Drug Administration is no exception, and this year especially it has challenged itself to find ways to enhance efficiency and update old regulations. Quality Digest has…

Dirk Dusharme
The Dec. 31, 2018 deadline looms for medical device companies that sell their devices in Canada. On that day, any company that sells medical devices to Canada will either need to hold an MDSAP certificate or show proof that they are on track to be MDSAP certified, or they won’t be able to sell…

Mike Richman
The future is the ultimate abstraction; anyone who has ever attempted to discern the nature of tomorrow by looking at the yesterdays leading up to today knows that prediction is a fool’s errand. That’s the unfortunate reality for weather forecasters, stockbrokers, sports bookmakers, political…
Ryan E. Day
One of the unique aspects of Finch Therapeutics is that although its product does not fall easily into any regulated category and thus is not FDA-approved, the company has been working closely with the agency for at least five years. The FDA has broad jurisdiction to regulate all health products,…