All Features
AssurX
Last month an investigative report revealed that the U.S. Food and Drug Administration (FDA) has millions of “hidden” serious injury and malfunctions reports on medical devices. According to the report from Kaiser Health News, “Since 2016, at least 1.1 million incidents have flowed into the…

Matthew M. Lowe
Despite the life science industry’s infatuation with modernity and trend chasing, even its most forward-thinking organizations have struggled to fully digitize and integrate their operations.
Yet, while the industry lags behind most other sectors in implementing business-streamlining digital…

Robyn Metcalf
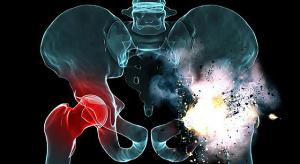
In an outbreak that has now run for more than 28 months, at least 279 people across 41 states have fallen ill with multidrug-resistant Salmonella infections linked to raw turkey products. Federal investigators are still trying to determine the cause. In response to food company recalls, more than …
NIST
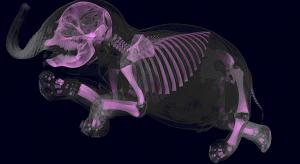
A new measurement approach proposed by scientists at the National Institute of Standards and Technology (NIST) could lead to a better way to calibrate computed tomography (CT) scanners, potentially streamlining patient treatment by improving communication among doctors.
The approach, detailed in…
Bruce Hamilton
Last February I had the opportunity to observe healthcare providers up close and personal at one the world’s premier hospitals. “Who Cares for the Caregivers?” was written from the perspective of a patient in a cardiac step-down unit, sympathetically watching caregivers as they grappled with many…

Shobhendu Prabhakar
Historically, conventional wisdom among business managers was that the higher the quality, the higher the cost. This perception still holds true today among a few business managers. Common sense also tells us the same thing, i.e., to create higher quality products or services, organizations will…

Hubert Gatignon
Health and economics are linked in more ways than just health insurance. When we look past the obvious, research shows us how brain scans, the gig economy, or even hospital queues are all part of the expanding domain of health economics.
Recently, professors and researchers from the Sorbonne…

Jon Speer
You arrive at work one morning, and there are FDA inspectors sitting in your waiting area. If you are lucky, you may be notified ahead of time that they’re coming, but otherwise, the US. Food and Drug Administration (FDA) is fully within its rights to show up unannounced at any time.
Because of…

Taran March @ Quality Digest
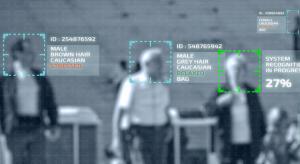
Life science companies are no strangers to data, so it would be easy to assume they are adept at making innovative use of huge amounts. Not necessarily. A tradition of rigorous scientific method and clinical trial hasn’t prepared them for the shifting inundation of big data or all its baffling…
Dirk Dusharme
For centuries, medical procedures, prescriptions, and other medical interventions have been based largely on experience—what is known about a set of symptoms. The doctor looks at those symptoms, tests you in various ways (blood tests, X-rays, MRIs), and interprets the results based on experience…
Zach Y. Brown
Imagine there was a store where there were no prices on items, and you never knew what you’d pay until you’d picked out your purchases and were leaving the shop. You might be skeptical that the store would have any incentive to offer reasonable prices.
This exact situation has become the norm in U…

Stephen McCarthy
I am thrilled to introduce Quality Digest’s special report, “Unlocking the Future of Life Sciences.” This series explores the last several decades of quality management within the life sciences industry. It begins with the genesis of early regulations, shows us how that led to the current state of…

Mike Richman
Great quality is pretty much the same everywhere, but the cost of poor quality is not equivalent from industry to industry. For example, it’s conceivable (but I hope not probable) that this article may turn out to be a real bomb, or worse, a complete snoozer. What’s the cost of that poor quality?…
Quality Digest
Within the life science industry, federal and industry regulations have prompted the need for compliance, and that trend has only increased in magnitude and complexity. Along with that has come technological solutions to enable both compliance and efficiency, without which life science…

Laurel Thoennes @ QD
Compliance to U.S. Food and Drug Administration (FDA) regulations has come a long way in the past 30 years. Here are the main changes. Have they affected your business?
1988: Food and Drug Administration ActOfficially establishes the FDA as an agency of the Department of Health and Human Services…

Dirk Dusharme
As the United States struggles with rising healthcare costs, reducing the amount of money pharmaceutical companies spend dealing with regulation, while at the same time meeting drug safety requirements, would seem to be competing interests.
The goal of any honest pharmaceutical company is to make…

Taran March @ Quality Digest
It’s been a year and a month since Stephen McCarthy switched C suites, moving from Johnson & Johnson, where he served as vice president of quality system shared services, to Sparta Systems, where he’s now vice president of digital innovation. His focus has switched as well.
At J&J, he…

Graham Freeman
Many industries have no clear boundary between safety and quality culture. In fact, they are often closely integrated. Quality failures and nonconformances that require rework have been correlated with increased accidents and recordable injury rates in manufacturing organizations. These injuries…

Teresa Purzner
Developmental biologist Matthew Scott and I went from purely basic biological research in our lab at Stanford University, to discovering a target for drug development, to identifying a drug for a pediatric brain cancer called medulloblastoma, to a clinical trial—all within five years and for just $…

Manfred Kets de Vries, Katharina Balazs
The global wellness industry is doing superbly, thank you very much. In recent years, it grew a healthy 12.8 percent, becoming a $4.2 trillion market. Whether the lives of wellness consumers are improving at a comparable rate is another matter altogether.
Wellness products and services run the…

Dirk Dusharme
In this episode we look at lessons learned (or not) from GE, the difference between ISO and FDA “requirements,” and this year's Baldridge recipients.
“GE’s Lessons Won’t Determine Whether You Succeed or Fail”
Does the success or failure of GE’s CEO really matter that much when it comes to how most…
Ryan E. Day
BioBridge Global (BBG) is a parent organization for four subsidiary organizations, three of which are involved in production activities, and they’re all around regenerative medicine, including blood components, clinical laboratory testing, and cell and tissue therapies. Organizations in the life…

Taran March @ Quality Digest
These days, even regulatory agencies must innovate if they expect to keep pace with the speed of doing business. The U.S. Food and Drug Administration is no exception, and this year especially it has challenged itself to find ways to enhance efficiency and update old regulations. Quality Digest has…

Dirk Dusharme
The Dec. 31, 2018 deadline looms for medical device companies that sell their devices in Canada. On that day, any company that sells medical devices to Canada will either need to hold an MDSAP certificate or show proof that they are on track to be MDSAP certified, or they won’t be able to sell…

Mike Richman
The future is the ultimate abstraction; anyone who has ever attempted to discern the nature of tomorrow by looking at the yesterdays leading up to today knows that prediction is a fool’s errand. That’s the unfortunate reality for weather forecasters, stockbrokers, sports bookmakers, political…