After a patient has a heart attack or stroke, doctors often use risk models to help guide their treatment. These models can calculate a patient’s risk of dying based on factors such as the patient’s age, symptoms, and other characteristics.
While these models are useful in most cases, they do not…
All Features

Kelvin Lee
Biopharmaceutical manufacturing uses living cells to produce therapies that treat diseases like cancer, diabetes, and autoimmune disorders. Manufacturing medicine using biology presents different challenges from the traditional chemical manufacturing processes that stamp out identical pressed pills…
Clinton Ballew
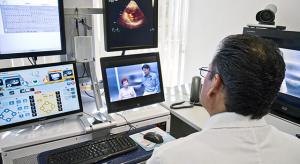
Legislative support is growing for the reimbursement of care delivery via telemedicine. The Centers for Medicare and Medicaid Services (CMS) and the Office of Inspector General have recently made final and proposed rule changes to stimulate greater use and access for telemedicine delivery. These…

Dirk Dusharme
What a year.
No matter your job, your industry, or your political beliefs, this year has been a heck of a ride. The (still ongoing) trade war with China, manufacturing gains (and losses), the 737 MAX, Hong Kong riots, North Korea, Brexit, impeachment. What a mixed bag of ups and downs that has…

As usual with Quality Digest’s diverse audience, this year’s top stories covered a wide range of topics applicable to quality professionals. From hardware to software, from standards to risk management, from China trade to FDA regulations. It’s always fun to see what readers gravitate to, and this…

Anat Amit-Eyal
Eric, a 40-something married father of three, runs a successful startup. Given his demanding career, he and his wife decided she would be a stay-at-home mum. Eric believed the attention he devoted to his family was adequate, and that he had fully harmonized his work as CEO and life as a family man…
Michael Millenson
In late November 1999, a TV producer called me about an alarming report that 44,000 to 98,000 Americans were being killed each year by preventable errors in hospitals, and another 1 million were being injured. Could that be true? Based on my research, I replied, the estimate seemed low.
The “To…

The QA Pharm
Weekly CGMP Quiz 1: Part 210 & 211 Subpart A General Provisions. Use with your team for training credit!
This is the first of eleven quizzes on CGMPs that will appear weekly on QA Pharm. Try it yourself, and use it as a discussion tool for your staff groups. Also, each quiz will have one…
Heather Thompson
Software as a medical device (SaMD) is a growing sector in medical device technology. Through the use of artificial intelligence and machine learning, SaMD has the power to influence health on a global scale as well as allow for personalization in medicine and life-saving therapies.
Medical device…

The company Grace Science was born through an inversion of the normal business sequence. Typically, if an entrepreneur launches a startup and it succeeds, the founders will create a nonprofit, declaring, “We want to give back.” In this case, the nonprofit spawned the startup.
The company’s…

Boris Liedtke
In May 2019, a California jury found Monsanto’s weed killer, Roundup, to be a “substantial factor” in the cancer suffered by a couple and ordered the U.S. agrochemical company to pay them $2 billion in damages. This was the third and largest verdict against Monsanto, now owned by German…

Lola Butcher, Knowable Magazine
Any patient scheduled for surgery hopes, and maybe assumes, that his surgeon will do a high-quality job. Surgeons know better. Nearly three decades of research have made clear that some hospitals and surgeons have significantly better outcomes than others.
Exactly how to measure the quality of a…

David Moser
Technology companies are frequently driven by their engineering processes. Of course product quality is regarded as most important, and that quality can be tested and measured with numbers and data. Such companies also frequently align their core identity with the engineering that belies their…
Jon Speer
When it comes to making medical devices, quality is key. That’s a concept that nearly every medical device professional agrees with, but what does it mean? Why is quality so important, and how should it be pursued? These are the questions that medtech executives and company stakeholders should be…

Samantha Maragh
I didn’t understand what people were asking me when I was a kid. The question would come in several different forms. Sometimes it was, “What are you?” Other times it was, “Where are you from?” I would answer with things I knew to be true, like, “I’m a girl,” or, “I’m a person,” or, “I’m from…

Daniel Hess
We all expect hospitals to be open and operating when we need them, but extreme weather events like hurricanes are a strain on resources and pose significant challenges for hospitals.
Closing a hospital is an extreme action, but several hospitals in Florida, Georgia, and South Carolina did just…

Sharona Hoffman
A career as a physician has traditionally been considered to be among the best vocations that talented students can pursue. That may no longer be the case. All too many doctors report that they are unhappy, frustrated, and even prepared to leave the profession.
That should worry all of us. The…

Peter Rose
On May 26, 2020, the new European Union Medical Device Regulation (MDR) will finally take effect. By that date, all Class I manufacturers wishing to continue their trading activities within the EU market must have effectively completed the transition from the previous medical device directive and…

Michael D. Williams
As I spoke recently with colleagues at a conference in Florence, Italy, about healthcare innovation, a fundamental truth resurfaced in my mind: the U.S. healthcare industry is just that. An industry, an economic force, Big Business. It is a vehicle for returns on investment first and the success of…
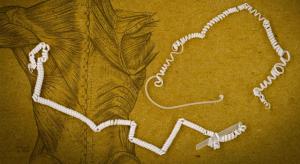
David L. Chandler
As a cucumber plant grows, it sprouts tightly coiled tendrils that seek out supports to pull the plant upward. This ensures the plant receives as much sunlight exposure as possible. Now, researchers at MIT have found a way to imitate this coiling-and-pulling mechanism to produce contracting fibers…
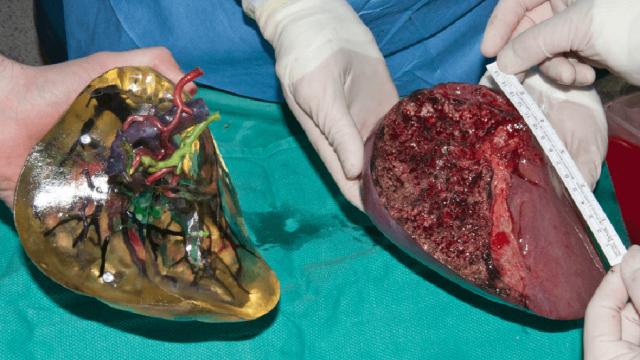
Sarah Webb, Knowable Magazine
This story was originally published by Knowable Magazine.
What you see in the image above is a lobe of a liver, times two. On the right, a flesh-and-blood one, removed from a transplant donor; and on the left, one created from plastic to represent bile ducts, arteries, and veins, which were laid…

Grant Ramaley
Although the “new approach” to regulating medical devices has always given more urgency to higher-risk medical devices, this is not the case for the European Medical Device Regulation (MDR). Class 1 medical devices must fully comply with the regulation by May 26, 2020, or be shut out of the region…

Jon Speer
This notion of risk-based processes within quality systems is something that has become part of our formal lexicon following the release of ISO 13485:2016, the globally harmonized standard for medical device quality management systems (QMS).
Well before these risk-based processes became a quality…

Matthew M. Lowe
While most business sectors have welcomed the efficiencies and benefits that cloud technologies and software-as-a-service (SaaS) offerings bring, the life sciences industry has been slow to embrace external cloud networks. Merely a decade ago, in fact, an International Data Corp. survey showed that…
Jon Speer
The European Medical Device Regulation (MDR) is a new set of regulations that governs the production and distribution of medical devices in Europe, and compliance with the regulation is mandatory for medical device companies that want to sell their products in the European marketplace.
If your…
