All Features

James J. Kline
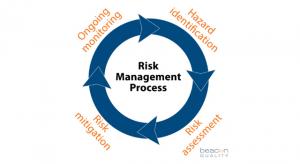
The term “risk-based thinking” (RBT) is familiar to those in the quality profession. This familiarity comes in part from its inclusion in ISO 9001:2015, the International Organization for Standardization (ISO) quality management system standard. Although numerous articles and several books have…

Kevin Price
In the world of risk management, maintenance of mission-critical equipment drives priorities and budgets. It is the ultimate test of proactive maintenance and smart decision making. Managing assets that “cannot be allowed to fail” is more than an emotionally charged mandate that forces managers…

Venkat Viswanathan, Shashank Sripad, William Fredericks
As electric cars and trucks appear increasingly on U.S. highways, it raises the question: When will commercially viable electric vehicles take to the skies? There are a number of ambitious efforts to build electric-powered airplanes, including regional jets and planes that can cover longer…
Shobhendu Prabhakar
Someone recently asked me why quality failures and safety incidents continue to occur despite organizations communicating their quality and safety visions to the workforce, developing and implementing quality and safety management systems, and campaigning day in and day out about quality and safety…
Ronda Culbertson
The AS9100 family of standards has completed very important updates, raising the business management quality bar again for aerospace and defense suppliers and OEMs. The transition to the new standards caught quite a few organizations somewhat flat-footed; particularly with the emphases on risk…
AssurX
Last month an investigative report revealed that the U.S. Food and Drug Administration (FDA) has millions of “hidden” serious injury and malfunctions reports on medical devices. According to the report from Kaiser Health News, “Since 2016, at least 1.1 million incidents have flowed into the…

Ryan E. Day
More and more, manufacturers are becoming the target of hackers, but what can they do about it, if anything? It seems every month, maybe even every week, we hear about some sort of data breach or cyberattack. Think Facebook, Google, and Marriott. As consumers we’ve almost become inured to the idea…

Beth Humberd, Scott Latham
Walmart recently announced it plans to deploy robots to scan shelves, scrub floors, and perform other mundane tasks in its stores as the retail giant seeks to lower labor costs.
Although the retail giant did not say which jobs, if any, might be lost as a result, the announcement—and the many more…
Paul Foster
When you look at standards like IATF 16949 or ISO 9001, the requirements boil down to two essential elements: improving customer satisfaction and reducing risk.
They go hand in hand because effective risk management means safer products and happier customers—and fewer problems for their suppliers…

Jeffrey Phillips
One of my favorite quotes comes from George Bernard Shaw, who said that all change in life originates from unreasonable people. Reasonable people, he said, will accept the status quo and change their lives to adapt to the status quo. Unreasonable people won’t. Unreasonable people force change…

Robyn Metcalf
In an outbreak that has now run for more than 28 months, at least 279 people across 41 states have fallen ill with multidrug-resistant Salmonella infections linked to raw turkey products. Federal investigators are still trying to determine the cause. In response to food company recalls, more than …

Morgan Sliff
Boeing has been rife with issues lately. While the recent Ethiopian Airlines crash has dominated headlines and elicited an FBI investigation into the company, another federal body has stated it will be keeping a closer eye on Boeing’s safety shortfalls.
Boeing is now in hot water with the U.S. Air…

Matthew M. Lowe
It’s human nature to resist change, and the life sciences industry is not exempt from a change-averse mindset. The proof: Life science organizations (LSOs) lag far behind counterparts in other sectors in implementing digital technologies that are designed to streamline business and manufacturing…

Theodoros Evgeniou
History indicates that major technological changes can take about half a century to go from the first lab drawings to society. Alan Turing first proposed the Turing machine, laying the foundations of computation, in 1936; the first general-purpose “Turing-complete” system was built in 1945, and “…

Sylvain Charlebois
Your own voice will likely become the most significant focus for food retailers and restaurants in the immediate future. Voice searches are increasingly becoming the norm. A recent study suggests that more than 50 percent of all online searches will be voice-activated by 2020.
To a lesser extent,…

Ryan E. Day
According to the International Labor Organization, around the world every day 7,600 people die from work-related accidents or diseases—that’s more than 2.78 million people every year. To address the issue, the International Organization for Standardization (ISO) has developed a standard, ISO 45001…

Stephen Rice, Scott Winter
In the wake of the Lion Air and Ethiopian Airlines crashes of Boeing 737 Max planes, people are thinking about how much of their air travel is handled by software and automated systems—as opposed to the friendly pilots sitting in the cockpit.
Older commercial airliners, such as the Beechcraft 1900…

Rachel Mann
With medical cannabis being legal in Canada since 2002, and recreational use becoming legal in 2018, Prime Minister Justin Trudeau’s promise to his country has caused a storm.
This legal framework has given Canadian companies the leverage to lead the cannabis industry to new heights. Not only…

Kelli Matthews
In a crisis, time is not on your side. A crisis creates a vacuum, an informational void that gets filled one way or another. The longer a company or other organization at the center of the crisis waits to communicate, the more likely that void will be filled by critics.
That’s exactly what’s…

Ismael Belmarez
Given the number of meetings most organizations have, you’d think everyone couldn’t help but be on the same page. Sort of a natural, automatic byproduct of spending so much time together. Nice idea, but not really true.
In fact, organizing is one of the most difficult things for an organization to…

Tom Taormina
Outsourcing is historically one of the most misunderstood concepts in quality management system (QMS) implementation and operation. Prior to ISO 9001:2015, the requirement for outsourced processes was limited to a few sentences in the standard’s clause 4.1. This article will present, through a case…

Shobhendu Prabhakar
Historically, conventional wisdom among business managers was that the higher the quality, the higher the cost. This perception still holds true today among a few business managers. Common sense also tells us the same thing, i.e., to create higher quality products or services, organizations will…

Chad Kymal
When we think about IT security, we typically think about the large hacks that were reported in the press. When viewed as a whole, we can understand the magnitude of lost data. It’s no surprise that these hacks are what come to mind when we think about information security.
The table below shows…

Oihab Allal-Chérif
In just five short months, two Boeing 737 Max 8 airliners crashed, killing a total of 346 passengers and crew members. Both crashes occurred shortly after takeoff, and the similarities between the two catastrophes raised fundamental questions about the aircraft’s safety. It was grounded by nation…

Claire Harbour, Antoine Tirard
Born to a Dalit family, Megha was raised in Southwest India and learned English at her convent school. As a child, she aspired to be a fashion designer or a cardiologist, but her parents insisted that she become an IT engineer. After four years of higher education, Megha found a job in the booming…