All Features

Etienne Nichols
The corrective and preventive action (CAPA) process is one of the most important elements within a medtech company’s quality management system (QMS). The goal of the CAPA system is to identify, address, and prevent systemic issues that could compromise product safety, regulatory compliance, and the…

Oak Ridge National Laboratory
Stronger than steel and lighter than aluminum, carbon fiber is a staple in aerospace and high-performance vehicles. Now, scientists at the U.S. Department of Energy’s Oak Ridge National Laboratory have found a way to make it even stronger.
ORNL researchers simulated 5 million atoms to study a …

Adam Galinsky, Maurice Schweitzer
Nano Tools for Leaders—a collaboration between Wharton Executive Education and Wharton’s Center for Leadership and Change Management—are fast, effective tools that you can learn and start using in less than 15 minutes, with the potential to significantly affect your success and the engagement and…

Stephanie Ojeda
Many companies are still clinging to paper-based and unconnected electronic processes, despite the clear disadvantages. Without modern tools like QMS software, these organizations risk compromising product quality, falling behind in compliance, and ultimately losing competitive ground.
In contrast…

Sanath Hegde
For quality heads, compliance officers, auditors, and engineering leaders, audits have been a time-consuming, resource-intensive process, yet necessary to build resilient operations, prevent costly failures, and maintain competitive advantage.
The question isn’t whether to audit, but how to…

Harish Jose
In this article I’m exploring the need for ethics in systems thinking using the ideas of Heinz von Foerster and Russell Ackoff. The two come from different traditions within systems thinking. Von Foerster comes from physics and second-order cybernetics, and Ackoff from operations research and…

Gleb Tsipursky
As companies and government agencies push forward with return-to-office (RTO) mandates, they risk exacerbating a workplace problem that many have failed to address adequately: gender discrimination.
New research from the University of Toronto’s Rotman School of Management highlights how in-person…
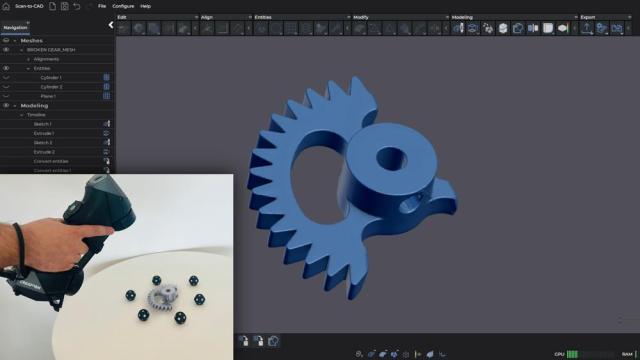
Creaform
Streamlining the transition from scan-to-CAD involves selecting the right tool set for reverse engineering. However, many software platforms force product designers to switch between multiple tools, disrupting their workflow and increasing the risk of errors. Furthermore, some advanced scan-to-CAD…

Prasant Prusty, Arundhathy Shabu
A global food safety and quality certification, BRCGS (British Retail Consortium Global Standards) initially focused on food safety but now comprises various sectors such as packaging, consumer products, and retail. It aims to ensure that businesses maintain high standards of safety and quality…
Etienne Nichols
As part of its effort to address the changing landscape around artificial intelligence (AI) in medical devices, the U.S. Food and Drug Administration (FDA) has recently released two new guidance documents on artificial intelligence-enabled device software functions (AI-DSF): • “AI-enabled device…
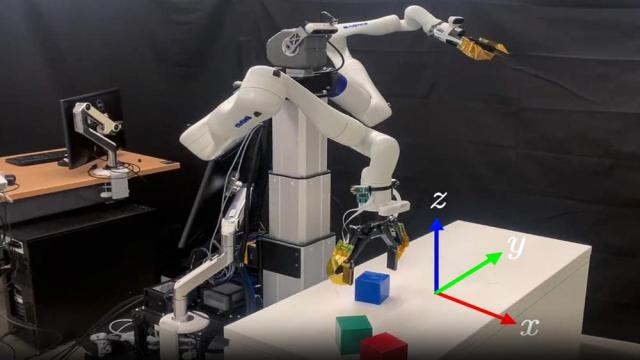
Adam Zewe
Ready for that long-awaited summer vacation? First, you’ll need to pack all items required for your trip into a suitcase, making sure everything fits securely without crushing anything fragile.
Because humans possess strong visual and geometric reasoning skills, this is usually a straightforward…

Cornelia C. Walther
On April 8, 2025, a driverless Zoox robotaxi misjudged an approaching vehicle, braked too late, and sideswiped it at 43 mph on the Las Vegas Strip.
One month later, the Amazon subsidiary issued a software recall on 270 autonomous vehicles and suspended operations while regulators investigated the…

Heidi Drafall
Anyone who has cracked their smartphone screen or had a rapid oil change knows that sometimes the OEM isn’t the most affordable or convenient service option. Consumer flexibility, paired with lower-cost, high-quality options, is logical, whether it’s in the consumer market or in healthcare.
The…

Jones Loflin
This past weekend I engaged in one of my favorite activities as a beekeeper. I got to catch a swarm of bees.
If you haven’t seen it before, it’s wild. About half of the bees leave with a queen and set up somewhere temporarily while they’re looking for their new permanent home. In the case of this…
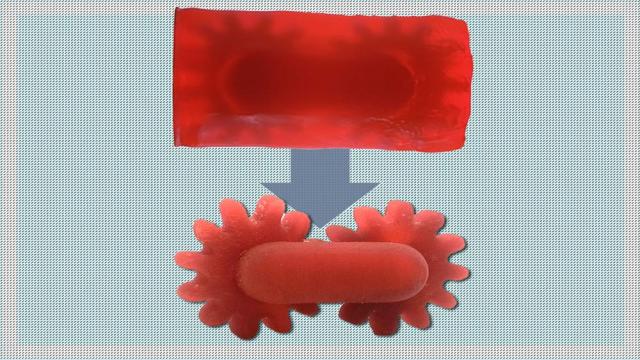
Jennifer Chu
Hearing aids, mouth guards, dental implants, and other highly tailored structures are often products of 3D printing. These structures are typically made via vat photopolymerization—a form of 3D printing that uses patterns of light to shape and solidify a resin, one layer at a time.
The process…

William A. Levinson
According to the U.S. News & World Report article “FDA Warns Sanofi of Manufacturing Irregularities at Key Facility” (Jan. 23, 2025), the pharmaceutical company Sanofi received a U.S. Food and Drug Administration warning letter “stating that FDA inspectors found irregularities with the facility…

Bryan Christiansen
The cornerstone of efficient industrial and facility management, maintenance, repair, and operations (MRO) cover all activities related to equipment maintenance, procurement, upkeep, and inventory management. This includes spare parts, consumables, lubricants, cleaning supplies, safety equipment,…

Harish Jose
I am a longtime admirer of George Spencer-Brown’s “Laws of Form.” In this article, I explore how his notion of reentry helps illuminate the paradoxes and blind spots in modern ideologies, especially the rise of xenophobia and extreme nationalism. These rigid ideologies depend on distinctions…
Angie Basiouny
Chatbots are everywhere. A fast-growing communication channel for brands, AI-powered chatbots are being deployed by companies to handle everything from booking travel to refunding purchases to helping shoppers choose the right outfit.
When done right, they can drive sales and provide positive…

Creaform
Ålö Agricultural Machinery (Ningbo) Co. Ltd., a wholly owned subsidiary of Ålö, is a leading manufacturer of loaders and implements for agricultural tractors.
With production facilities in four countries, and customers in more than 50, Ålö holds approximately 30% of the world market for tractor…

Ariana Tantillo
A team from MIT Lincoln Laboratory has built and demonstrated wide-band selective propagation radar (WiSPR), a system capable of seeing out various distances at millimeter-wave (mmWave or MMW) frequencies. Typically, these high frequencies, which range from 30 to 300 gigahertz (GHz), are employed…
Matt McFarlane
One of the key findings in Greenlight Guru’s 2025 Medical Device Industry Report was that economic uncertainty is playing a large role in the decisions medical device companies make this year.
The report surveyed more than 500 medical device professionals across quality, regulatory, product…

Stephanie Ojeda
Every day, quality leaders face a variety of production and process issues. Although some problems are easy to fix, others require deeper investigation, such as using a 5 Whys analysis or fishbone diagram. But then there are the stubborn, recurring issues that can lead to quality issues, increased…

Annette Aquino
To meet the precise requirements associated with semiconductor manufacturing, integrated circuit (IC) packaging and assembly providers must navigate complex quality certification processes, such as those for the well-known ISO 9001 and ISO 13485 standards. Another important certification for…

ISO
Occupational health and safety (OHS) is often brushed aside as a checkbox exercise—something assigned to compliance officers or forgotten in day-to-day operations. But this mindset comes at a cost. Every year, millions of people suffer injuries, illnesses, or worse, simply because their workplace…