All Features

Stephanie Ojeda
When organizations implement an enterprise quality management system (EQMS), the instinct is often to begin with high-visibility processes like corrective and preventive action (CAPA) or supplier quality. While these functions are critical, starting there can be a misstep. Without the right…

William A. Levinson
My June 2025 article, “How to Avoid FDA Warning Letters,” points out that inadequate corrective and preventive action (CAPA) is a major reason for warning letters, and also introduces the role of failure mode effects analysis (FMEA) in preventing trouble in the first place. The U.S. Food and Drug…

Mike Figliuolo
I had a great conversation with a friend of mine. He was bemoaning the fact that his company was almost completely dependent on one huge customer. He saw the inherent risks in that relationship but confessed that his organization had a bad habit it couldn’t kick. It had succumbed to the addiction…

Lexi Sharkov
We’d be willing to bet your key collaborators aren’t all in the same building. Your team members, contract partners, clients, and suppliers are likely scattered across the globe. That makes collecting physical, “wet ink” signatures nearly impossible and turns digital approvals into a daily…

MasterControl Inc.
Ninety days to implementation vs. 12 to 18 months with traditional systems: That’s not just an incremental improvement—it’s a complete reimagining of what’s possible in life sciences quality management.
In the highly regulated life sciences industry, quality management system (QMS) implementations…

Lexi Sharkov
When an issue arises, it’s important to take quick action. Whether that means launching a software patch, pulling a batch, or halting the use of a reagent, it’s critical to tackle the immediate problem.
But just as critical as “How do we fix this?” is “How do we make sure this doesn’t happen again…

NIST
In the wake of Covid-19 and widespread wildfires, demand skyrocketed for air cleaners, machines that could remove potentially harmful particles from the air in a home. Manufacturers responded by producing a wider variety of air cleaner devices designed for single rooms.
The main purpose of an air…

Brookhaven National Laboratory
Plan a route, grab some snacks, and fuel up. Engineers and scientists have been sending massive magnets from U.S. Department of Energy (DOE) national labs on cross-country road trips.
Magnets are at the heart of many scientific instruments at the DOE’s Brookhaven National Laboratory. They aren’t…

Quality Digest, Jason Chester
Today, manufacturing is largely shaped by supply chain volatility, complex labor dynamics, and—like most global industries—the rise of AI. Adopting AI technologies on the shop floor can help manufacturers minimize operational costs, mitigate risk, and optimize processes, which drives efficiency and…
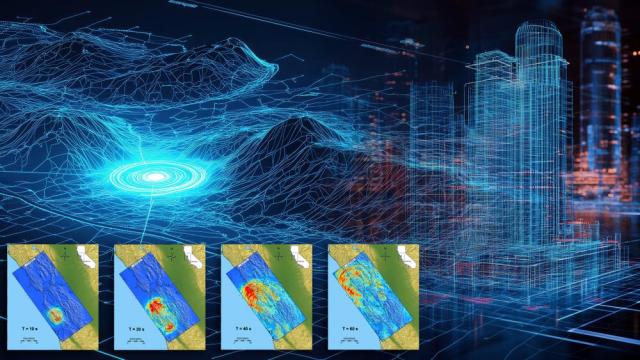
Oak Ridge National Laboratory
Simulations still can’t predict precisely when an earthquake will happen. Still, with the incredible processing power of modern exascale supercomputers, they can now predict how they will happen and how much damage they will likely cause.
Imagine a colossal earthquake strikes the California coast…

Walter Nowocin
Software selection, implementation, and ongoing maintenance are critical stages in the life cycle of biomedical software systems such as asset and calibration management platforms. Yet few industry resources provide detailed, practical guidance for managing these processes effectively.
One notable…

Judy Fainor
What if your quality system could detect and initiate corrective actions for equipment deviations before they affect product quality?
It’s a compelling vision—and one that’s becoming increasingly achievable through AI-enabled automation. But let’s be clear: We’re not there yet. What we do have is…

Cornelia C. Walther
Sarah, a marketing director at a Fortune 500 company, recently celebrated her team’s 40% productivity increase after implementing AI-powered content generation tools. Her seasoned copywriters now produce campaigns in hours rather than days, while AI handles routine social media posts and email…

Mike Regan
In July 2024, CrowdStrike rolled out a software update that crashed more than 8 million Windows systems worldwide. The faulty release disrupted hospitals, grounded flights, halted banking operations, and affected government services. Comparable to a major cyberattack, the incident caused more than…

William A. Levinson
A vital concept from the chemical process industry, management of change (MOC) relates primarily to safety. It means that whenever we change a factor in a cause-and-effect diagram (e.g., machine, material, manpower, method, measurement, environment, or any other factor), we create risks of…

Cindy Mielke
Over the years, there have been many developments in systems and technologies that have helped industries redefine how businesses are structured. These changes, especially in sectors like manufacturing and distribution, have led to new roles and responsibilities for employees.
However, often with…

Jake Walton
Imagine you’re a student trying to pass a challenging class, one where the entire grade rests on the big test at the end of the semester. Fortunately, the professor handed out a syllabus that outlines exactly what will be on that final exam. Better still, you can also find a posted list of common…

Sachin Waikar
Take two aspirin and call me in the morning: If only prescribing medications were as simple as that.
In reality, the prescription process involves many players and steps. Details must be accurately spelled out, interpreted, and double-checked to ensure patients get the correct drug and dosage.
“…

Dottie DeHart
Work-life balance is tough, and for most of us, it’s starting to feel like a thing of the past. Companies are demanding longer hours and increased productivity. Remote workers are being called back to the office, and the ones still at home are stuck in “always on” mode. With layoffs happening left…

ISO
Security configuration management (SCM) is a critical concern for organizations and a fundamental part of many cybersecurity frameworks. Consider this scenario: A team member tweaks a hardware setting on their personal laptop to boost software performance. However, this change causes unforeseen…

Susan Robertson
Productivity looks good on paper. It’s measurable, visible, and in many organizations, it’s worshipped. But here’s the problem: Productivity isn’t the same as progress.
Many cultures confuse motion with momentum. Leaders celebrate packed calendars, rapid responses, and efficiency hacks, but often…

Kamran Sayrafian
A few years ago, I heard on the news that many people were being hospitalized with a condition of excess fluid in the lungs, called pulmonary edema. It’s common in elderly patients. Pulmonary edema is dangerous and can lead to breathing difficulties and lung failure. Because it has the potential to…

Creaform
Karbonius Composites specializes in manufacturing precision molds and composite components across various sectors, including automotive restoration and high-performance customization. Founded in 2008 and based in La Coruña, Spain, the company’s production capabilities cover every step needed to…
Kristin Burnham
Companies that adopt industrial artificial intelligence see productivity losses before longer-term gains, according to new research.
Organizations have long viewed artificial intelligence as a way to achieve productivity gains. But recent research about AI adoption at U.S. manufacturing firms…
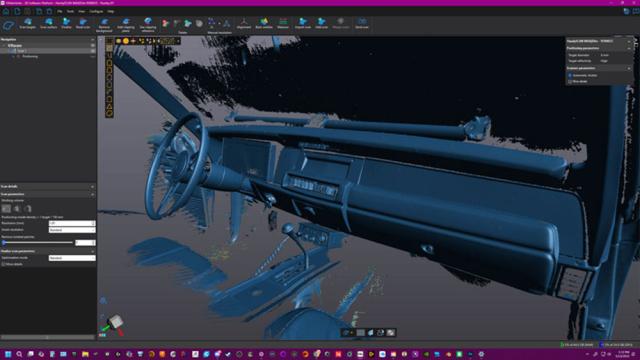
Creaform
Based in Tampa, Florida, Starr Creations, a powerhouse in fiberglass design and fabrication, is known for turning ambitious automotive visions into reality. Jesse Starr, creative director and 3D designer, founded the company with a passion for racing and innovation.
Starr Creations has been the…