In the healthcare sector, precision isn’t just a requirement. It’s a necessity where the margins for error are perilously thin, and the consequences of inaccuracy can be grave. At the heart of this precision lies the unassuming yet critical load cell, a device whose reliability is foundational to…
All Features

Dawn Bailey
The Center for Organ Recovery & Education (CORE), a 2019 Baldrige Award recipient, is a nonprofit organ procurement organization (OPO) in Pittsburgh with a federally designated service area encompassing a population of 5.5 million in western Pennsylvania, West Virginia, and one county in New…

James Chan
Management of change (MOC) is a defined process that organizations establish and follow to ensure health, safety, and reduction of risk during periods of change. MOC systems and policies are essential for companies that undergo significant updates to their facilities, personnel, equipment, or…

Gleb Tsipursky
Generative AI, when harnessed correctly, has the potential to revolutionize the way companies operate, innovate, and compete. But the question that arises is how businesses can effectively tap into this potential. The answer lies in setting up an AI center of excellence that combines IT with…

ISO
Trust makes the world go ’round. Without it, democracies crumble and relationships suffer. The same goes for organizations and businesses: Without the trust of their customer base, they simply can’t succeed.
Trust, however, is never a given. Like respect, it must be won. In an ever-evolving…

Saurabh Joshi Shripad
Risk assessment and continual process verification (CPV) are fundamental regulatory requirements for pharmaceutical companies to ensure drug safety, efficacy, and quality. While risk assessment involves analyzing, mitigating, communicating, and monitoring risks that could ultimately affect patients…

Ferdinando Fioretto
Artificial intelligence’s capacity to process and analyze vast amounts of data has revolutionized decision-making processes, making operations in healthcare, finance, criminal justice, and other sectors of society more efficient and often more effective.
However, with this transformative power…

Douglas C. Fair, Scott A. Hindle
In less than two months we will celebrate the 100th anniversary of the invention of the control chart, a tool most often associated with statistical process control (SPC). Considering SPC from our modern perspective made us ask, “Is SPC still relevant?”
It’s a question asked within the purview of…

Stephanie Ojeda
Companies today implementing automated compliance management systems are motivated by a wide variety of factors.
For many, it’s about reducing manual labor hours required to execute quality processes—and achieving greater efficiency and effectiveness in their operations. For others, the priority…

Patrick Gale
Medical equipment is a necessary yet substantial investment for any health system. Making strategic decisions about these assets can be daunting in the face of shifting patient demand, financial uncertainty, and fast-changing cybersecurity risks.
Because clinical assets account for an average of…

Denise Robitaille
The buzz has begun. ISO 9001 is being revised. There hasn’t been a revision in about 10 years, so it’s due—if not overdue. Still, there are individuals who don’t understand the justification or the purpose of the revision. After all, it’s a perfectly good standard. So, what’s up?
It’s worth…

Jennifer King
The cost of poor quality can be devastating to business: Failed quality control costs manufacturers anywhere between 15–20% of their total profits on average, and as much as 40% for some, the ASQ reveals. Businesses with successful quality programs, on the other hand, can benefit from increased…

William A. Levinson
What do quality and productivity have to do with World War III, which we all hope will never happen? The answer is everything. A massive loss of American manufacturing capability between 1945 and 2024 has conceded enormous advantages to aggressor nations that might be inclined to break the peace.…
Alonso Diaz, Maria DiBari
The U.S. Food and Drug Administration (FDA) emphasizes the importance of being prepared for device recalls.
FDA product recalls are on the rise in the post-pandemic era. There has been a clear upward trend from 2021 through 2023, and medical devices ranked the highest of all product types. (See…

Stephanie Ojeda
Implementing an automated compliance management solution is a mammoth undertaking with high stakes and potentially high returns for those who navigate the process successfully.
Get it right and you could save thousands of labor hours, avoid millions of dollars in compliance issues, and free up…

William A. Levinson
In his Quality Digest article published in February 2023, Michael Mills1 reported that the next version of ISO 9001 will add to clause 4.1, “Understanding the organization and its context” the words, “the organization shall determine whether climate change is a relevant issue.”
Although nothing in…

Stephanie Ojeda
As U.S. Supreme Court Justice Louis D. Brandeis famously wrote, “Sunlight is said to be the best of disinfectants.”
In the field of quality, internal audits are the equivalent of sunlight. Like spring cleaning, internal audits provide the opportunity to bring process issues into the open before…

Grant Ramaley
When it comes to protecting anyone or anything from harm caused by something manufactured, grown on a farm, or rolling down a highway or a runway, quality is of utmost importance. Our trust forms the backbone of what we expect from our food, cars, planes, medical devices, and protection of the…

Jamie Bihary
An internal audit can be an overwhelming prospect, especially if you’re new to a company or internal auditing in general.
The MedTech space is huge, and even the standards that are meant to help, like ISO 13485:2016, cover a lot of ground.
So, if you’re part of the audit team in your company, and…

James Chan
Reactive maintenance is a form of physical asset management in which maintenance is only performed on equipment or machinery when it malfunctions or fails entirely. Sometimes referred to as run-to-failure maintenance or breakdown maintenance, reactive maintenance strategies provide the most basic…

Denise Robitaille
ISO 9001 has begun its revision process. In the next few months, all eyes will be riveted on that arena as everyone seeks to anticipate the changes and what they’ll augur for their own quality management systems. The attention is not undeserved.
Equally important but with considerably less…

Jamie Fernandes-ETQ
Generative AI took the world by storm in 2023, from the classroom to the film studio, and the writer’s bench to the White House. Enterprises and creative industries worked to figure out how to leverage it in their operations, while classrooms and government entities struggled to govern its use.
In…
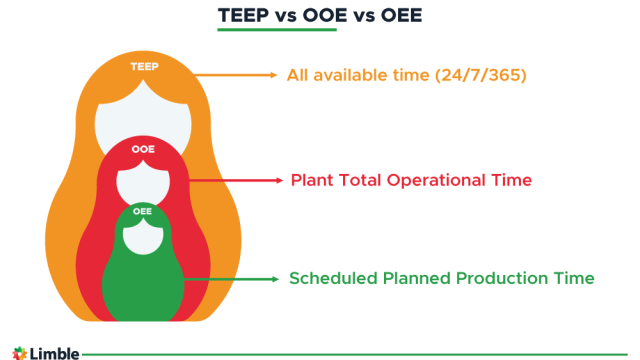
James Chan
In manufacturing industries, calculating overall equipment effectiveness (OEE) offers deep insights into the performance of production processes and enables data-driven improvements.
Determining OEE is a standard method for assessing the productivity of production lines compared to their…

Erin Vogen
Utility companies play an essential role in our communities, supporting our well-being and quality of life. But providing always-on services that rely heavily on machinery, technology, and other assets can pose a challenge. In the dynamic and demanding world of utilities, mastering the art of asset…

Matthew Barsalou
The FMEA (failure modes and effects analysis) turned 75 years old in 2024. However, a look at the literature may paint a different picture. Both the origin year of FMEAs and the name of the organization that developed FMEAs seem to vary among authors. Much of the literature on FMEAs is inconsistent…
