All Features

ISO
Healthcare administrators find themselves at the fore of a demanding and transformative field, where the pursuit of excellence in patient care is nonnegotiable. In a health industry landscape facing evolving regulations, escalating costs, and an increasing emphasis on patient outcomes, the need for…

Mike Figliuolo
I’m going to take over the world! It’s really fun to say that. It’s even more fun to take action toward that goal.
Our world has gotten smaller. Way smaller. Globalization is an unstoppable trend. But as they say, the trend is your friend, so why not take advantage of it? I don’t care how big or…

Kate Zabriskie
“I don’t understand. This hotel has no restaurant, no concierge, and no valet. It’s not at all what I expected. The place had no standards.”—Guest at a roadside motel
“I showed her the picture of Jennifer Aniston and asked for the same haircut. She cut my hair, but I still don’t look a thing like…

Stephanie Hinton
If you’re conducting a clinical investigation of a medical device in a European Union member state, you will be required to submit a clinical investigation report (CIR) along with a summary of the CIR to that member state.
The European Union Medical Device Regulation (EU MDR) lists this as one of…

Kelley Jacobsen
In the wake of the Covid-19 pandemic, medical device supply chains are one of the top priorities for health system leaders. Amid supply chain disruptions during the pandemic, hospitals scrambled to find enough devices to keep up with unprecedented demand. The global crisis revealed gaps in standard…

Etienne Nichols
Supply chain management is crucial to any medtech company’s ability to deliver safe, effective, and high-quality devices to their customers.
But as anyone in the industry can tell you, consistently getting the products and services you need to manufacture your devices is harder than it sounds. In…
Chris Bush
Untitled Document
The U.N. recognizes privacy as a fundamental human right, and nowhere is this more important than in medical data. That’s why both the U.S. and the EU have regulations in place that govern the collection, storage, and use of patient data in healthcare.
In the U.S., there is the…
Jennifer Chu
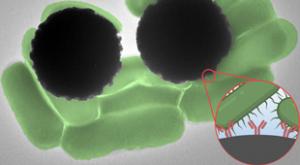
Getting blood test results can take anywhere from a day to a week, depending on what a test is targeting. The same goes for tests of water pollution and food contamination. And in most cases, the wait time has to do with time-consuming steps in sample processing and analysis.
Now, MIT engineers…

NIST
Magnetic resonance imaging (MRI) machines can clearly view non-bony parts of the body—soft tissue such as the brain, muscles, and ligaments—as well as detect tumors, making it possible to diagnose many diseases and other conditions. However, the powerful magnets in conventional MRI machines make…

Matthew M. Lowe
Let’s start with a definition of Industry 4.0, keeping in mind that we’re rapidly approaching Industry 5.0. Industry 4.0 is an era marked by enhanced digitization and the increased connectivity of smart technologies. Where Industry 5.0 is more values-driven, it will require the technology of…

William A. Levinson
Starbucks’ implementation of artificial intelligence coffee makers1 offers a simple and ideal case study that can illustrate the synergy between efficiency, wages, profits, and inflation. Even if we don’t know the actual cost figures, we can use some hypothetical numbers to demonstrate how higher…

Peter Fader
As an organizational leader, you’ll be very familiar with your company’s key financial statements and monthly management reports. But what do you really know about the people who pull out their wallets and pay for your products and services? In The Customer-Base Audit: The First Step on the Journey…

Adam Zewe
Imagine purchasing a robot to perform household tasks. This robot was built and trained in a factory on a certain set of tasks and has never seen the items in your home. When you ask it to pick up a mug from your kitchen table, it might not recognize your mug (perhaps because this mug is painted…

Lindsey Walker
In the quickly changing industrial landscape, firms continue to place a high premium on safety. Innovative approaches to improving industrial safety have been made possible by technological advancements. One particularly revolutionary option is computerized maintenance management system (CMMS)…
Etienne Nichols
On Feb. 23, 2022, the U.S. Food and Drug Administration (FDA) released its proposed rule for the new Quality Management System Regulation (QMSR). The proposed QMSR will be the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s Quality System Regulation (QSR…

Kate Zabriskie
‘Who designed this convoluted process? A monkey could have done a better job.”
“Why do I have to come in and talk to someone? This whole transaction could be handled online. Frustrating!”
“I dread going there. The parking lot is impossible to navigate, you fill out what seems like 8,000 forms…

Etienne Nichols
Your company probably has an internal process for a large purchase like an eQMS. In midsize-to-large medtech companies, you’ll likely find this process in the finance department, or perhaps in a dedicated purchasing department operating under finance’s umbrella.
1. Start by learning about your…

Kari Miller
Since 2010, citations for insufficient corrective action and preventive action (CAPA) procedures have been at the top of the list of the most common issues within the U.S. Food and Drug Administration (FDA) inspections, particularly for the medical device industry. Issues can occur while…

Etienne Nichols
Amedical device company is expected to deliver innovative, life-changing devices while ensuring compliance and achieving true quality. This task bears loads of responsibility—all of which must be kept and documented within your quality management system (QMS).
A QMS contains everything that…

FABTECH
Manufacturers understand that their businesses won’t grow if their workforces don’t grow along with them. That’s why the talent shortage in the metal fabrication industry continues to be a pressing concern—and three-quarters of respondents in the National Association of Manufacturers’ 2023 First…

Aaron Smith
A successful company can’t run without happy and motivated employees. One way you can achieve that is by improving your employees’ uptime. Uptime refers to your employees’ freedom to pursue personal and occupational growth without the burden of preventable injuries. Here is everything you need to…

Stephanie Ojeda
Design controls are a frequent citation in 483 observations and warning letters from the U.S. Food and Drug Administration (FDA). In fact, the agency has noted a large proportion of past recalls that could have been prevented with design controls.
FDA guidance also makes an explicit link between…

Gleb Tsipursky
The unemployment rate is surprisingly low, at 3.7%, shocking economists who expected a slowdown in hiring and rising unemployment rate. Frontline work, such as healthcare, led job growth. Frontline workers are in high demand, and the competition for their services is fierce. Yet wage growth cooled…

Etienne Nichols
The goal of your MedTech company’s supplier management process should be to ensure a consistent supply of high-quality parts and components that conform to your specifications.
But achieving that goal is easier said than done, and it depends heavily on whether you take a risk-based approach to…

Jon Finerty
In today’s highly competitive business environment, a trusted supply chain that functions seamlessly is essential. Even small errors can affect the entire supply chain, resulting in lost revenue, unsatisfied customers, and irreparable damage to your brand.
Now more than ever, companies need the…