All Features

John Courtney
Customers are the lifeblood of any business. Without them, there would be no profits to distribute, no people to serve, and no reason to continue operating. To keep your business running on a path to growing success, you need to offer a customer experience that will make customers choose your brand…

Claudine Mangen
Work has become an around-the-clock activity, courtesy of the pandemic and technology that makes us reachable anytime, anywhere. Throw in expectations to deliver fast and create faster, and it becomes hard to take a step back.
Not surprising, many of us are feeling burned out. Burnout—which often…

Jill Roberts
Florida’s outbreak of listeria has so far led to at least one death, 22 hospitalizations, and an ice cream recall since January 2022. Humans get sick with listeria infections, called listeriosis, from eating soil-contaminated food, undercooked meat, or dairy products that are raw or unpasteurized.…

Gregory Way
Drugs don’t always behave exactly as expected. While researchers may develop a drug to perform one specific function that may be tailored to work for a specific genetic profile, sometimes the drug might perform several other functions outside of its intended purpose.
This concept of drugs having…

Adam Zewe
Physicians often query a patient’s electronic health record for information that helps them make treatment decisions, but the cumbersome nature of these records hampers the process. Research has shown that even when a doctor has been trained to use an electronic health record (EHR), finding an…

Karina Montoya
Close to 9 million people in India suffer from hepatitis C. If left untreated, the virus leads to cirrhosis or liver damage, which eventually causes death from organ failure or cancer. On average, a 50-year-old man in India with asymptomatic liver damage who doesn’t receive treatment is expected to…

Alixandra Barasch
If you’ve ever played Wordle, learned a new language on Duolingo, or worked out with Peloton, you may be familiar with daily app notifications that nudge you to keep at it—or risk breaking a streak of consecutive efforts. Do you or don’t you heed the clarion call?
If you do, you’re in good company…

Tom Rish
Your design history file (DHF) is one of the most critical components of your QMS. That’s because the DHF should contain all the product development documentation for a specific medical device. Its purpose is to show regulatory bodies and internal stakeholders that you appropriately followed the…

Patricia Santos-Serrao
The pharmaceutical industry has seen significant upheaval and disruption during the past several years. These changes are due in part to the impacts of Covid—for example, interruptions in the supply chain and overwhelming market demand for shortened production times.
They are also being driven by…
Mike John
This article has been republished with permission from Medical Plastics News.
While ISO 13485 sets the standard for quality management systems (QMS) in medical device manufacturing, metrology is often treated as an afterthought and used simply to validate products and detect defects at the end of…

Gleb Tsipursky
The pandemic has made organizations aware of the need for a new C-suite leader, the CHO, or chief health officer. This has been driven by recognizing the importance of employee health for engagement, productivity, and risk management, along with lowering healthcare insurance costs. At the same time…

Ann Brady
Safer food, better health: This was the theme of World Food Safety Day (June 7, 2022), and it’s obvious, is it not, that access to safe food is vital for life and health? The challenge in today’s world is how to achieve this. Global food systems, already under pressure before the pandemic, are now…

Del Williams
The use of membrane technology as a processing and separation method in the food industry is gaining wide application for demineralization, desalination, stabilization, separation, deacidification, purification, and reducing microbial load.
Perhaps the most obvious application for membrane…
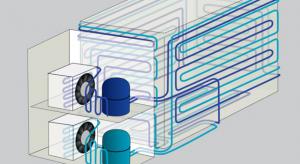
Jamie Steiner
Ultra-low temperature freezers became popular due to the storage of Covid-19 vaccines, but they have been important components of laboratories for many years. There’s a lot, however, to think about—quality, productivity, maintenance, different types of technology, warranties, etc. And if you end up…

Prashant Yadav
During the past two and a half years, we’ve seen unparalleled innovation and private-public collaboration in the global fight against Covid-19. The rapid development and rollout of new vaccines, diagnostic tests, and therapeutics have saved millions of lives.
However, these developments haven’t…
David Stevens
The United States has more than 6,000 hospitals, and each one has thousands, if not tens of thousands, of clinical assets, such as imaging machines, ventilators, and IV pumps. Managing this equipment becomes a mighty task when hospital staff must handle the monitoring, repair, and maintenance of…
Kari Miller
Quality management is essential to the growth and performance of any organization. It’s a valuable resource in the effort to ensure that products and services satisfy the highest quality requirements and deliver positive customer results.
Pharmaceutical manufacturers must ensure that the…
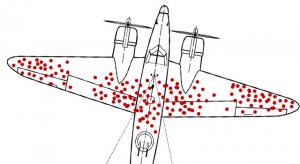
William A. Levinson
Quality-related data collection is useful, but statistics can also deliver misleading and even dysfunctional results when incomplete. This is often the case when information is collected only from surviving people or products, extremely satisfied or dissatisfied customers, or propagators of bad…

Kevin Ketels
The conditions that led to a shortage of baby formula were set in motion long before the February 2022 closure of the Similac factory tipped the U.S. into a crisis.
Retailers nationwide reported supplies of baby formula were out of stock at a rate of 43 percent during the week ended May 8, 2022,…

The Un-Comfort Zone With Robert Wilson
In a recent column, I wrote about the power of suggestion. I stated, “When our subconscious mind is exposed to a constantly repeated message, it’s going to penetrate unless we are cognizant of it.” Becoming conscious of indoctrinating media messages is important, but recognizing your own internal…
Merilee Kern
Since the dawn of civilization, humans have used natural remedies for their healing properties. Some of the same treatments are still used by billions around the world, based largely on anecdotal evidence and lore. Clinical research on natural treatments is lacking due to costly clinical trials,…

Dario Lirio
By now, it’s no secret that good clinical practice (GCP) guidelines used by FDA inspectors are expanding. These GCP guidelines are developed by the International Conference on Harmonization. The ICH last revised its GCP document, called ICH E6(R2), in 2016. It will be releasing a new version in…

Alexander Khomich
The digital transformation of healthcare is under the influence of trending technologies, from IoT devices to AI algorithms. Some healthcare providers are just getting acquainted with innovations. Others (93%, according to Accenture) are already actively implementing and creating software solutions…

Katarina Bennich
Ever found yourself hitting the wrong button and then flipping through the manual in a frenzy, trying to figure out how to get that thing to stop doing what it’s doing? If your answer is yes, you’ve been an unfortunate victim of bad user experience (UX).
UX is defined as all aspects of a product,…

Gary Shorter
Predictive and prescriptive insights driven by data analytics have risen to prominence as tools that can help research teams cut the time, complexity, and cost of clinical trials. At the same time, these insights can enhance the quality of a study and accelerate new drugs to market. But to uncover…