All Features
Creaform
Based in Tampa, Florida, Starr Creations, a powerhouse in fiberglass design and fabrication, is known for turning ambitious automotive visions into reality. Jesse Starr, creative director and 3D designer, founded the company with a passion for racing and innovation.
Starr Creations has been the…

Lexi Sharkov
They may seem simple, but SOPs (standard operating procedures) are surprisingly tricky. On the surface, they describe how a specific process is carried out at your organization. Pretty straightforward, right?
In reality, there are a lot of requirements for writing a good SOP, and auditors have a…

FOCO
Metal foundries are under intense pressure. Soaring energy bills now eat more than 30% of production budgets. Tough carbon regulations, such as the EU’s Carbon Border Adjustment Mechanism, reduce profit margins.
Foundries also struggle with costly scrap loss through melting and meeting tough…
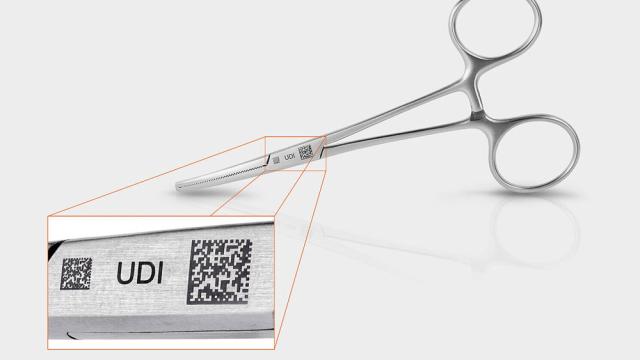
FOBA
In medical technology, safety is paramount. Every step in the manufacturing process must meet the highest standards, from patient protection to regulatory compliance. Because direct part marking plays a key role in ensuring traceability and unique device identification (UDI) conformity, it’s…

Creaform
Since its founding in 1974, Intercontrôle has been a leader in France’s nuclear energy sector, ensuring the safety of reactor components through automated nondestructive testing (NDT).
As part of the Framatome group, the company develops its own inspection equipment, oversees logistics and…

NIST
A rapidly growing category of drugs called protein-based biotherapeutics can be used to treat cancers and genetic and autoimmune disorders. These drugs, which usually take the form of large protein molecules, are manufactured by growing living cells that are genetically engineered to produce the…

Creaform
The Bott Group designs vehicle and operating equipment as well as workplace systems. From its headquarters in Gaildorf, Germany, and two production sites in Bude, England, and Tárnazsadány, Hungary, Bott Group designs and manufactures work environments for mobile and stationary manual work…

Stephanie Ojeda
Global-scale events have tested the bounds of supply chain systems. The coronavirus, for example, made it clear how critical an efficient supply chain is for continuity and survival. It’s a real-world example of how important it is to have an enterprisewide system that uses a quality management…

Brookhaven National Laboratory
Ateam of scientists across several U.S. Department of Energy national laboratories has unraveled how light and a previously unknown form of certain nickel-based catalysts together unlock and preserve reactivity. This research, described in the journal Nature Communications, could potentially…
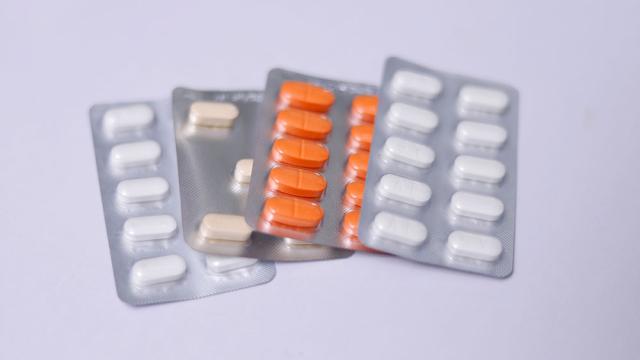
Nimax
Pharmaceutical serialization practices are on the rise and have progressively become a worldwide standard as a result of stringent regulations in various of markets, including the United States, European Union, China, and Argentina. Recent estimations found that by 2022 serialization practices had…

Ariana Tantillo
A new security screener that people can simply walk past may soon be coming to an airport near you. Last year, U.S. airports nationwide began adopting HEXWAVE, a commercialized system based on microwave imaging technology developed at MIT Lincoln Laboratory. HEXWAVE addresses a new Transportation…

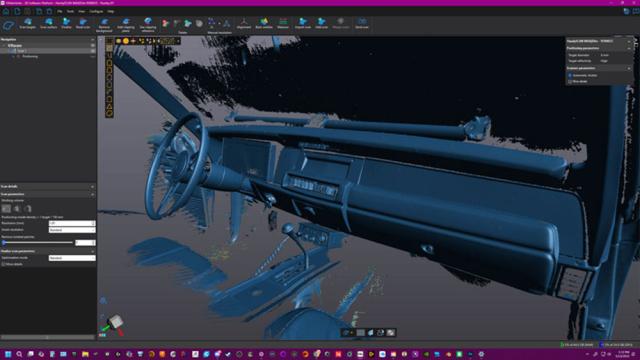
Creaform
Known for building rugged telehandler equipment that delivers reliable performance in the most demanding environments, Xtreme Manufacturing places a strong emphasis on quality in every stage of production.
To uphold its commitment to quality when inspecting large weldments, Xtreme needed a…

Robyn Coward
In life sciences, every decision carries weight—and speed to market is an ever-present consideration. Scientific innovation is moving faster than ever, yet regulatory demands are growing more complex, and supply chain fragility has become the new normal. Within this volatile landscape, the role of…

Jennifer King
Although patient safety is paramount in healthcare settings, about 1 in 10 patients is harmed in healthcare, and more than 3 million deaths occur due to unsafe care, says the World Health Organization (WHO). The reality is hospitals and healthcare facilities face numerous challenges in managing…

Etienne Nichols
Good supplier management is one of the most important methods of building a safe and effective medical device. A single device may be made up of dozens of parts and components coming from several different suppliers, and many medical device companies outsource the manufacturing of their device to a…

Akhilesh Gulati
Complacency won’t show up on a control chart. But its damage is real. Can AI and systems thinking help us detect it and respond before trust is lost?
As customer expectations evolve, one question remains: Are customers still at the core of your company’s operations?
Back in 1999, a simple but…

Harish Jose
I’m further exploring the notion of models and mental models. We often speak of mental models as though they’re neat packages of knowledge stored somewhere in the mind. These models are typically treated as internal blueprints and as simplified representations of the world that help us navigate and…

Michael Mills
From April 10 through July 3, 2025, ISO (International Organization for Standardization) had the opportunity to vote on a draft update to the global standard ISO 9000 “Quality management—Fundamentals and vocabulary.” ISO 9000 is the companion document to the more widely known ISO 9001. It contains…

John Tschohl
Why do customers patronize one company over another? Many of you might say that the quality and price of the products or services are key factors. But while those things might play into a purchasing decision, they aren’t the most important consideration.
So, what is? Customer service. How you and…

Hexagon Manufacturing Intelligence
Did you know that shutdowns, turnarounds, and outages (STOs) can consume up to 50% of a plant’s annual maintenance budget? That’s according to a report by the Boston Consulting Group.
STOs are among the most complex and high-stakes events in industrial operations. They’re costly, especially when…

Patrick Willemson
The European Union has taken a leading role in shaping a variety of data and AI regulations. One of its most recent initiatives, the Ecodesign for Sustainable Products Regulation (ESPR), extends this regulatory momentum into the manufacturing sector. Under this new regulation, manufacturers and…

Bryan Christiansen
Facility teams are constantly balancing urgent repairs, preventive tasks, asset tracking, and compliance, all while ensuring smooth day-to-day operations. But when processes are manual, fragmented, or unclear, even simple tasks can spiral into delays, miscommunication, and wasted time.
The…
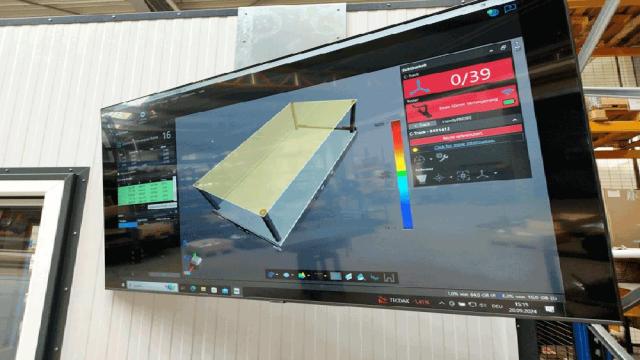
Creaform
Brosius GmbH is a trusted partner in metal processing for a wide range of companies in the mechanical and plant engineering industries, offering comprehensive services under one roof. Brosius ensures that every part it manufactures in its 10,000 m² state-of-the-art production hall meets or exceeds…

Harish Jose
Readers of my blog might be aware that I appreciate the nuances of cybernetic constructivism. Cybernetic constructivism rejects the idea that we have access to an objective reality. It doesn’t deny that there’s an external reality independent of an observer. However, we don’t have direct access to…

Mark Hembree
The 2025 Major League Baseball season certainly started with a bang—at least for the New York Yankees. At Yankee Stadium against the Milwaukee Brewers, the Bronx Bombers blasted an MLB-record 15 home runs in the first three games, including nine homers in a 20-9 bludgeoning of the Brewers in the…