All Features
Rob Matheson
Carrying your smartphone around everywhere has become a way of life. In doing so, you produce a surprising amount of data about your role in the economy—where you shop, work, travel, and generally hang out.
Thasos Group, founded at MIT in 2011, has developed a platform that leverages those data,…

Dirk Dusharme
In our April 13, 2018, episode of QDL, we talked about anti-hacker robots, data privacy, and new product introduction.
“HoneyBot Lures in Digital Troublemakers”
MIT nerds come up with a tasty target for IoT hackers. But this one fights back.
“We Don’t Care About Data Privacy”
Privacy, schmivacy.…

Marin Hedin
Limiting first-year medical residents to 16-hour work shifts, compared to “flexing” them to allow for some longer shifts, generally makes residents more satisfied with their training and work-life balance. It also makes their training directors more dissatisfied with curtailed educational…

Knowledge at Wharton
America’s healthcare system has been on the examining table lately: from the tortuous battle over the Affordable Care Act, to Senator Bernie Sanders’ bill to allow low-cost prescription drugs in from Canada, to the intriguing announcement in January that Amazon, Berkshire Hathaway, and JPMorgan…

Dirk Dusharme
On April 10, 2018, Facebook co-founder and CEO Mark Zuckerberg testified before Congress regarding the unauthorized sharing of 87 million Facebook users’ personal data, vacuumed up by data research company Cambridge Analytica. There were pointed questions regarding Facebook’s lack of transparency…

David Schwinn
After my recent extended illness, I was surprisingly shocked to reemerge into organizational life in its broadest terms. Frequently, I engaged in the organizational lives of my students, my friends, my colleagues, and my own workplace.
Everywhere I looked, I found: • Unhappy customers who, after…

Mike Richman
During this past Friday’s episode of Quality Digest Live, our weekly web TV show, QD editor in chief Dirk Dusharme and I covered stories about the gig economy and the skills gap and workforce shortages within manufacturing, especially as it relates to metrology, which is the science of measurement…

Chad Kymal
ISO 45001 is the much-anticipated, first ISO-based international occupational health and safety (OH&S) standard. The International Organization for Standardization (ISO) has tried twice and failed in the past to create an international OH&S management system standard. Although there are a…
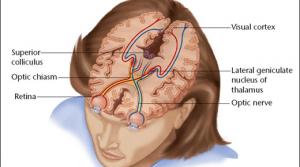
Malvina Eydelman
The U.S. Food and Drug Administration’s (FDA) Breakthrough Devices Program is beginning to show important results for patients since it was established in late 2016 under the 21st Century Cures Act to help patients gain timely access to breakthrough technologies.
Consider Second Sight Medical…

Debashis Sarkar
The cheating at Kobe Steel shook not just Japan but the entire manufacturing world. As Kobe Steel CEO Hiroya Kawasaki revealed, about 500 companies had received its falsely certified products, which affected not only those companies but also its entire supply chain. However, the issue at Kobe was…

Chip Bell
The coolest birthday present I ever received was a gift from my wife a number of years ago; it was a white 1962 Mercedes-Benz 220 sedan reasonably well-restored. But the classy antique car, with its deep fenders and leather seats, turned out to be a real lemon. That’s about all I remember about…

Annette Franz
Do you feel like you’re not making the progress in your customer experience (CX) transformation efforts that you thought or hoped you would by now?
You started years (not months—it’s a journey) ago, but you don't think your organization has evolved.
What’s the reason for that?
I’ve seen several…
Ian Setliff, Amyn Murji
The current 2017–2018 flu season is a bad one. Hospitalization rates are now higher than in recent years at the same point, and infection rates are still rising. The best line of defense is the seasonal influenza vaccine. But H3N2 viruses, like the one that’s infecting many people this year, are…

Mary Beth O’Leary
Matt Bianchi had a problem. As chief of the division of sleep medicine at Massachusetts General Hospital, he needed a better way to diagnose sleep disorders. Typically, a patient seeking a diagnosis needs to come into a sleep lab and be attached to a number of devices. This setting is hardly…

Davis Balestracci
The Individuals chart is the “Swiss Army knife” of control charts. It usually approximates the supposedly “correct” chart under most conditions, and its use is much easier to understand and explain. It can also save you a major side trip into the swamp of unnecessary calculation minutiae,…

Curt Redden
We all seem to get it by now—more engaged employees perform at a higher level. The organizations that get their strategy right in this area provide a superior customer experience, have lower levels of employee churn, higher morale, and ultimately much higher financial performance. Their customers…

Willie L. Carter
What differentiates a lean-thinking organization from a traditional one? Basically, the lean-thinking organization is grounded in the answers to two simple questions: “What do my customers value?” And, “What organization and work processes inside my company will most directly deliver that value?”…

Stephen McCarthy
Cost of quality (CoQ) is certainly not a new topic. It was first described in 1956 by American quality control expert Armand V. Feigenbaum in a Harvard Business Review article. As you likely already know, CoQ consists of four categories: internal and external failures, and appraisal and prevention…

Knowledge at Wharton
When Tide and other detergent manufacturers developed colorful, convenient pods designed to be tossed into washing machines and dishwashers, they never expected teenagers would try to eat them. But what was dubbed the “Tide pod challenge” quickly went viral, with teens posting videos of themselves…
Morten Wendelbo, Christine Crudo Blackburn
Flu season in the United States typically peaks in February, but this year’s outbreak is already one of the worst on record. As of Jan. 6, 2018, 20 children have died from the flu, and overall mortality caused by the virus is already double that of last year’s.
One reason the flu is so severe…

George Hall
Every year, would-be suitors spend lots on cards, nice trinkets, flowers, and even chocolates, trying to win the attention of their sweetheart or crush. It can be a dangerous game of risk and chance, quite often resulting in disappointment for one or both parties.
This is, I believe, most likely…

John Bell
I have written more than a 100 blog posts about leadership, strategy, and culture. Within that portfolio are several accounts of business reinvention and transformation. Yet it was only a few months ago that I composed my first post on another type of reinvention: personal reinvention. My own. …

AssurX
Recent FDA warning letters indicate that many drug manufacturers do not have their manufacturing in a state of current good manufacturing practices (CGMPs) control. During the first half of 2017, the FDA cited adulterated products and insanitary conditions as the two most common violations in drug…

Wendy Wood
Employers have a stake in their staff’s health. It’s not just a matter of keeping health insurance premiums in check which is a consideration in countries without universal healthcare. It’s also about maximizing employee engagement and productivity, and even happiness.
Promoting health habits is…

Julia Russell
Retailers and brands convened in New York recently to experience the National Retail Federation’s Retail’s Big Show, and one of the biggest topics on attendees’ minds was technology. From automation to personalization to social marketing, the growing importance of technology in the shopping…