All Features
Jon Speer
When it comes to making medical devices, quality is key. That’s a concept that nearly every medical device professional agrees with, but what does it mean? Why is quality so important, and how should it be pursued? These are the questions that medtech executives and company stakeholders should be…

Steven Brand
The food industry is evolving rapidly, with consumers demanding quality, authenticity, and transparency from food manufacturers. And they’re not just demanding it; they’re “voting with their dollars,” supporting companies that align with their personal beliefs. To keep up with consumer demand—and…

Peter Rose
On May 26, 2020, the new European Union Medical Device Regulation (MDR) will finally take effect. By that date, all Class I manufacturers wishing to continue their trading activities within the EU market must have effectively completed the transition from the previous medical device directive and…

Stephen McCarthy
In our constantly evolving, data-rich universe, collecting, interpreting, and understanding process data can be tricky. But it is increasingly important if we want to maintain sustainable quality across product development and manufacturing processes. This challenge is particularly evident in the…

Jennifer Lopez
Globalization of the medical device market as well as its supporting supply chains continues to increase year after year. This has forced regulatory bodies to grapple with finding a way to narrow the gap between international and domestic regulation. In spring 2018 the United States Food and Drug…
The FDA has announced an end to the alternative summary reporting (ASR) program for medical device manufacturers and will make the data publicly accessible.
The ASR program originally launched in 2000 when device manufacturers sought an “alternative summary” reporting exemption. ASR permitted…

Grant Ramaley
Although the “new approach” to regulating medical devices has always given more urgency to higher-risk medical devices, this is not the case for the European Medical Device Regulation (MDR). Class 1 medical devices must fully comply with the regulation by May 26, 2020, or be shut out of the region…

Jon Speer
This notion of risk-based processes within quality systems is something that has become part of our formal lexicon following the release of ISO 13485:2016, the globally harmonized standard for medical device quality management systems (QMS).
Well before these risk-based processes became a quality…

Matthew M. Lowe
While most business sectors have welcomed the efficiencies and benefits that cloud technologies and software-as-a-service (SaaS) offerings bring, the life sciences industry has been slow to embrace external cloud networks. Merely a decade ago, in fact, an International Data Corp. survey showed that…
Jon Speer
The European Medical Device Regulation (MDR) is a new set of regulations that governs the production and distribution of medical devices in Europe, and compliance with the regulation is mandatory for medical device companies that want to sell their products in the European marketplace.
If your…
AssurX
Last month an investigative report revealed that the U.S. Food and Drug Administration (FDA) has millions of “hidden” serious injury and malfunctions reports on medical devices. According to the report from Kaiser Health News, “Since 2016, at least 1.1 million incidents have flowed into the…

Matthew M. Lowe
Despite the life science industry’s infatuation with modernity and trend chasing, even its most forward-thinking organizations have struggled to fully digitize and integrate their operations.
Yet, while the industry lags behind most other sectors in implementing business-streamlining digital…

Matthew M. Lowe
It’s human nature to resist change, and the life sciences industry is not exempt from a change-averse mindset. The proof: Life science organizations (LSOs) lag far behind counterparts in other sectors in implementing digital technologies that are designed to streamline business and manufacturing…

Jon Speer
You arrive at work one morning, and there are FDA inspectors sitting in your waiting area. If you are lucky, you may be notified ahead of time that they’re coming, but otherwise, the US. Food and Drug Administration (FDA) is fully within its rights to show up unannounced at any time.
Because of…
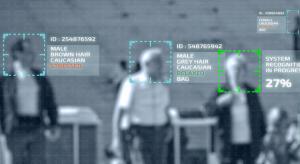
Dirk Dusharme @ Quality Digest
For centuries, medical procedures, prescriptions, and other medical interventions have been based largely on experience—what is known about a set of symptoms. The doctor looks at those symptoms, tests you in various ways (blood tests, X-rays, MRIs), and interprets the results based on experience…

Nicole Radziwill, Graham Freeman
In 2013, thousands of consumers in the United Kingdom (UK) and Ireland bought, prepared—and ate—beef lasagna, hamburgers, and frozen dinners. What they didn’t know is what they were actually putting in their mouths.
Although a burger is only required by law in that region to contain 47-percent…

Kelly Kuchinski
Imagine building a brand over decades. Hundreds of millions of dollars invested in design and development. Sponsorships with celebrity athletes and professional and college teams. Leading-edge marketing making your company one of the top 20 brands in the world. It only takes one incident to unravel…

Mike Richman
Great quality is pretty much the same everywhere, but the cost of poor quality is not equivalent from industry to industry. For example, it’s conceivable (but I hope not probable) that this article may turn out to be a real bomb, or worse, a complete snoozer. What’s the cost of that poor quality?…
Quality Digest
Within the life science industry, federal and industry regulations have prompted the need for compliance, and that trend has only increased in magnitude and complexity. Along with that has come technological solutions to enable both compliance and efficiency, without which life science…

Laurel Thoennes @ Quality Digest
Compliance to U.S. Food and Drug Administration (FDA) regulations has come a long way in the past 30 years. Here are the main changes. Have they affected your business?
1988: Food and Drug Administration ActOfficially establishes the FDA as an agency of the Department of Health and Human Services…

Dirk Dusharme @ Quality Digest
As the United States struggles with rising healthcare costs, reducing the amount of money pharmaceutical companies spend dealing with regulation, while at the same time meeting drug safety requirements, would seem to be competing interests.
The goal of any honest pharmaceutical company is to make…

Graham Freeman
Many industries have no clear boundary between safety and quality culture. In fact, they are often closely integrated. Quality failures and nonconformances that require rework have been correlated with increased accidents and recordable injury rates in manufacturing organizations. These injuries…

Dirk Dusharme @ Quality Digest
We interview Stanley Chao, author of Selling to China: A Guide for Small and Medium-Sized Businesses (iUniverse, 2018), about the impact of the current U.S.-China trade war. Does China really care, and where do U.S. multinationals go from here? Also, a quick look at Conformance Manager, a web-based…

Mike Richman
One of our favorite things on our show is to welcome guests, either via Skype or live in the studio. And this week, we were joined by three of our great partners. Here’s a closer look:
Interview: Nicole Radziwill of Intelex
Radziwill is quality manager and data scientist along with the presenter of…

Dirk Dusharme @ Quality Digest
In this episode we look at lessons learned (or not) from GE, the difference between ISO and FDA “requirements,” and this year's Baldridge recipients.
“GE’s Lessons Won’t Determine Whether You Succeed or Fail”
Does the success or failure of GE’s CEO really matter that much when it comes to how most…