All Features

Donald J. Wheeler, Al Pfadt
In memory of Al Phadt, Ph.D.
This article is a reprint of a paper Al and I presented several years ago. It illustrates how the interpretation and visual display of data in their context can facilitate discovery. Al’s integrated approach is a classic example not only for clinical practitioners but…

Etienne Nichols
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) issued a new draft guidance, “Content of Human Factors Information in Medical Device Marketing Submissions,” that provides recommendations for the inclusion of human-factors information in marketing submissions.
The drafted guidance is…

Kate Zabriskie
‘They’re hit or miss: Sometimes the service is marvelous. Other times it’s simply meh. I’m afraid to recommend the place because I can’t trust them to deliver.”
“Maybe I’m just boring, but I don’t like surprises. They’re great one day and disappointing the next. I don’t need to be delighted. I…

Trupti Dhere
The healthcare industry is known for rapidly adopting advanced technologies that offer improved treatment for various diseases. Consequently, digital twin technology in healthcare has gained popularity during the past few years, owing to the range of advantages it offers.
Digital twin technology…

Greg Kihlström
Customer loyalty and lifetime value are highly prized in today’s competitive environment. Smart businesses know that a major point of competitive advantage (or failure) can be the type of customer experience they provide.
The need to balance what customers want with what the business needs to…

Etienne Nichols
In a highly regulated industry like medical technology, manufacturing processes must undergo either process verification or process validation to ensure they’re consistently producing the correct result. The question is, which one should you use?
Verification and validation are two different…

Jon Munnery
When choosing to spend money, customers will likely investigate the brand behind the name to get an idea of whom they’re building ties with as well as an impression of service quality. Customer reviews must point to a genuine interaction with the company to be valuable and influence purchasing…

Liza Dzhezhora
Having appeared in the early 2000s, connected health technologies have gradually become a game changer in the healthcare industry. Healthcare providers that have embraced smart medical IoT solutions reduce costs, improve patient experience, and ensure preventive care.
The trend is not fading away…

Lisa Apolinski
A not-so-surprising fact, according to HubSpot: 65 percent of consumers state that the experience they encounter on a website is a “very important” factor in recommending a brand. If that statistic’s not enough, HubSpot also reported that 75 percent of consumers expect new technologies to be used…
Shabnam Azimi
Consumers who have a personality that scores high in terms of openness—such as being open to new adventures and intellectually curious—have better success at spotting fake reviews than other personality types, according to our recently published research. Extroverted people, on the other hand, tend…

Del Williams
For owners and operators in the agricultural and food-processing industries, Jan. 1, 2022, was the deadline for completing a dust hazard analysis (DHA) for existing facilities in accordance with Chapter 7 of the National Fire Protection Association’s Standard 61 (2020) for the Prevention of Fires…

Tess Malone
Imagine messaging an artificial intelligence (AI) chatbot about a missing package and getting the response that it would be “delighted” to help. Once the bot creates the new order, it says it’s “happy” to resolve the issue. Afterward, you receive a survey about your interaction. But would you be…
Scott Trevino
Nearly a quarter of surveyed healthcare cyberattack victims experienced increased mortality rates following a data breach, and more than half reported poorer patient outcomes due to longer hospital stays and delayed procedures. Healthcare has faced the highest average data breach cost—more than $10…
Amy Brown
Listening to customers is critical for healthcare organizations to ensure they’re delivering high-quality care to their patients. Sure, the traditional methodology of doing so via surveys can increase customer retention and profitability. But much like evolving from analog to digital, there’s a…
Gleb Tsipursky
One of the key stakeholders in stakeholder capitalism is the employee. You could argue that the employee is the key stakeholder, because without employees you’d have no stakeholders at all. This is why employers need to stay aware of today’s health environment and its effect on their employees.…

In a press statement released on Jan. 6, 2023, the European Commission reported the adoption of a proposal to allow more time to certify medical devices to mitigate the risk of shortages. The proposal introduces a longer transition period to adapt to new rules, as foreseen under the Medical Devices…

Bhushan Avsatthi
The very nature of healthcare construction and its specific infrastructural and functional needs pose significant challenges to the architectural, engineering, and construction (AEC) sector. Crucial hospital spaces such as operation theaters and critical care centers need fail-proof connections to…

Scott Ginsberg
We’re told the cardinal rule of the internet is, “Never read the comments.” This catchphrase is used to warn users of the toxic parts of the internet. One minute you’re sharing an article, photo, or video that you’re proud of. The next moment, dozens or even hundreds of comments snowball into a…

Andrey Solin
The other day I watched House of Gucci—a biographical movie directed by Ridley Scott about the Tuscan dynasty representing the famous luxury fashion house. The story is based on a nonfiction book written by Sara Gay Forden, The House of Gucci: A Sensational Story of Murder, Madness, Glamour, and…

Angie Basiouny
In the hustle of a busy hospital emergency department, teams of doctors and nurses react quickly to determine whether a patient needs to be admitted, referred, or released. Providing such complex care requires a high degree of skill and seamless teamwork, the kind that usually comes from years of…
Doug Folsom
Unpatched vulnerabilities remain a target of cyberattacks, and an ever-present risk for healthcare organizations. Medical devices pose an additional burden because patches are frequently unavailable for medical devices. So, dealing with the potential threat isn’t usually straightforward. The stakes…

Harry Hertz
The saying is nothing new: The customer is always right. Customers come first. We’ve heard these adages for a long time. And we’ve questioned them for almost as long. Those of you who know me know that I’ve certainly been doing that for a long time!
Two recent experiences brought this topic back…

Del Williams
With the threat of contamination from harmful pathogens such as salmonella, listeria, and e. coli a continual concern, food processors are seeking to protect not only the public but also their companies’ bottom lines from the massive costs, reputational damage, and greater regulatory scrutiny…
Anton Ovchinnikov
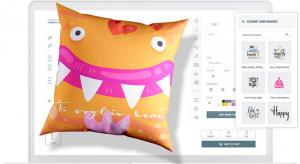
In the age of mass production, the demand for customization is increasing. Customers prefer products catered to their individual needs and preferences over standard items—albeit at a cost.
Fortunately, recent advances in information technology, logistics, and advanced manufacturing processes such…

Dirk Dusharme
In 2010 a medical device scandal in France set the stage for a new European Union medical device regulation that, according to most experts in the medical device community, may cause more damage than the problem it was intended to address. An unreasonable deadline, lack of notified bodies to…