All Features
Henrik Hulgaard
The phrase “supply chain” became part of the everyday vernacular during the pandemic, as supply chain issues seemed to affect everyone's life—from toilet paper to automotive components. Supply chain problems are well known to cause disruptions with product delivery, particularly in the complicated…

Laurie Guest
Everyone’s heard of it by now: “Quiet quitting” is the freshly coined phrase to describe the age-old behavior of not quite leaving one’s job entirely but rather opting to no longer go above and beyond. It’s service fatigue to the extreme, risking not just customer satisfaction but also staff…

Adriana Lynch
The decade of the 2020s has given businesses—and the consumers who count on them—quite a ride. Neither tea leaves nor the best efforts of analysts could have predicted the impact that the one-two punch of a pandemic and roller-coaster economy would have on both markets and marketplaces.
Consumers…
Alex Waddell, Diki Tsering, Peter Bragge, Paul Kellner
Emergency medical workers, already at increased risk for burnout compared to other professions, continue to be challenged by the fallout of Covid-19.
Stretched to the breaking point by increased workloads, highly contagious and acutely ill patients, and limited resources, workers’ risk factors for…

Quy Huy
In September 2022, Boeing agreed to pay $200 million for charges that it misled investors about two crashes of its 737 Max aircraft that killed 346 people. The penalty imposed by the U.S. Securities and Exchange Commission is small change compared to the $2.5 billion shelled out by the plane maker…

Matt Fieldman
Customer experience, or “CX,” is all the rage in marketing circles nationally. Customer experience refers to how a customer experiences your company at every point of their buying journey—from marketing to sales to customer service, and everywhere in between. It can be tangible actions, such as…

Dave Gilson
Like most of us, lawyers think they can be impartial when they rate other people’s work. “They say, ‘Who writes a brief doesn’t matter. A brief is a brief; it stands on its own merit,’” explains Lori Nishiura Mackenzie, the lead strategist for diversity, equity, and inclusion at Stanford Graduate…

James Gaines, Knowable Magazine
In 2004, the United States’ Defense Advanced Research Projects Agency (DARPA) dangled a $1 million prize for any group that could design an autonomous car that could drive itself through 142 miles of rough terrain from Barstow, California, to Primm, Nevada. Thirteen years later, the U.S. Department…

Tom Taormina
We live in a rural area, and many of our nonconsumables are purchased online. We’re deceivingly spoiled living near an Amazon fulfillment center because we can order an item on Saturday, and it arrives on Sunday. To me, this is a masterful logistical feat of filling an order, getting it into the…

Kevin Kanimyar
Amidst the rise of conscious consumerism, corporate social responsibility (CSR) programs are fast becoming an essential part of any business. With about $20 billion spent every year by Fortune 500 companies alone, businesses around the world are working to integrate CSR programs into their business…

Jon Speer
Medical device product development and risk management are often treated as entirely separate processes. Sure, there is usually acknowledgement and understanding that these two processes are related. But it is important to realize that product development and risk management share more than that.…

Dirk Dusharme
Every company wants to succeed, but not all can say they meet the current requirements to do that. More than a focus on capital, business plans, or staff, a successful business in 2022 must operate digitally. Yet for the 45 percent of small and medium-sized businesses (SMBs) that still rely on…

Laurie Flynn
AStanford Medicine-led study has found that borrowing certain billing- and insurance-related procedures from other countries could lead to policies that drastically lower healthcare costs in the U.S.
The new study, published in the August edition of Health Affairs, compares costs of healthcare…

Matthias Gouthier
The ISO Foresight Trend Report highlights global trends across multiple industries that will shape strategic decision making for a better future. Drawing upon these insights, ISO reflects on some of the potential areas for standardization work. In a series of feature articles, we unpack some of the…
Bendta Schroeder
The first step in choosing the appropriate treatment for a cancer patient is to identify their specific type of cancer, including determining the primary site: the organ or part of the body where the cancer begins.
In rare cases, the cancer’s origin can’t be determined, even with extensive testing…
Dwayne Duncum
The workplace has changed forever, having gone through a revolution similar to the Industrial Revolution. Our workplaces are diverse, complex, and frequently changing. If we take any lesson from the Covid pandemic, it’s that the way we work, where we work, and how we work have fundamentally shifted…

Jason Bradshaw
As a busy leader or business owner, you’re faced with a seemingly endless to-do list to keep your business operating, as well as an ever-increasing list of ideas about how to improve it. However, I suggest you throw out those hundred-plus to-do items and ideas and instead focus on the experience…

Etienne Nichols
I know what you’re thinking. You’ve got a medical device prototype that the FDA has categorized as Class I. You’re ready to push forward to manufacturing or marketing the device, since there are no formal requirements for design controls. “So why would I waste time on design controls?”
The fact is…

Mona Rhodes
Standards of customer service rose in 2022, and your business needs to keep up if you want to be successful in the digital age. Each customer interaction is crucial, especially since PWC revealed that 32 percent of customers will stop doing business with a brand when they experience poor service.…

Gleb Tsipursky
Organizations need to incorporate constructive feedback from stakeholders to survive disruptions amid today’s turbulent economy. Securing constructive feedback is critical in helping you find which decisions are working and which ones aren’t. Yet, many organizations fail to engage effectively with…

Catherine Barzler
Falls are a serious public health issue that result in tens of thousands of deaths annually while racking up billions of dollars in healthcare costs. Although there has been extensive research into the biomechanics of falls, most current approaches study how the legs, joints, and muscles act…

Yosef Ayzencot
Starting a business is a costly investment. According to U.S. Bureau of Labor statistics, more than half of businesses fail within the first five years of opening. Adding to this pressure were the nationwide staffing challenges during the “Great Resignation” and then the “Great Reshuffle.” This…
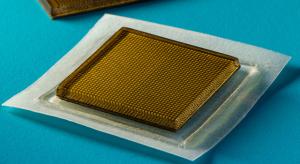
Jennifer Chu
Ultrasound imaging is a safe and noninvasive window into the body’s workings, providing clinicians with live images of a patient’s internal organs. To capture these images, trained technicians manipulate ultrasound wands and probes to direct sound waves into the body. These waves reflect back out…

Grant Ramaley
The FDA Quality System Regulation (QSR) 21 CFR Part 820 was written in 1997 to harmonize with ISO 13485:1996. The goal was to relieve some of the burden of manufacturers having to meet two different criteria, the FDA’s and ISO 13485.
But by 2003, ISO 13485 had changed so significantly that the FDA…

John Courtney
Customers are the lifeblood of any business. Without them, there would be no profits to distribute, no people to serve, and no reason to continue operating. To keep your business running on a path to growing success, you need to offer a customer experience that will make customers choose your brand…