All Features
Jay Desai
The presidential symposium at this year’s annual meeting of the Child Neurology Society of America in early October in Kansas City raised many eyebrows: The first presentation focused on burnout rates among neurologists around the country.
Many of my colleagues felt that this was an inappropriate…

ISO
A new version of ISO 31000:2009—“Risk management” is due to be unveiled early next year. As the threat of risks grows for governments, organizations, and the public alike, how can the new, streamlined standard help to make our future more secure?
Ten years ago, the boardrooms of banks and…
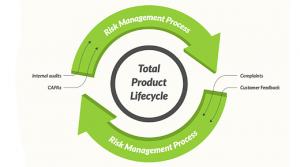
Jon Speer
What exactly is a risk-based quality management system (QMS)? This is a timely topic to get into. In 2016, ISO 13485—“Medical devices”—“Quality management systems” was updated, and one of the key concepts presented is the idea of a risk-based QMS.
Historically, regulations have almost exclusively…

Scott Shackelford
Hackers around the world are attacking targets as diverse as North Dakota’s state government, the Ukrainian postal service, and a hospital in Jakarta, Indonesia. Unfortunately, many governments—in the developing world, and even cash-strapped states and local communities in the United States—lack…

Jason Furness
You have defined what you want as an outcome of the change program; you have looked at how to understand your financial statements and how to use them to assess options. You have looked at the obstacles that lay in your path. Now we are going to start to look at your business, specifically.
Why…

Fred Schenkelberg
The term “Weibull” in some ways has become a synonym for reliability. Weibull analysis = life data (or reliability) analysis. The Weibull distribution has the capability to describe a changing failure rate, which is lacking when using just mean time between failures (MTBF). Yet, is it suitable to…

Jeremy Straub
Some people are afraid that heavily armed artificially intelligent robots might take over the world, enslaving humanity—or perhaps exterminating us. These people, including tech-industry billionaire Elon Musk and eminent physicist Stephen Hawking, say artificial intelligence technology needs to be…

Olympus
Sponsored Content
High-volume manufacturers need fast, nondestructive testing methods to help avoid material mix-ups and to meet customer quality requirements. Quality assurance (QA) inspectors are responsible for evaluating incoming raw materials by determining their elemental makeup and…

Kemper Lewis
President Trump has long talked about reinvigorating U.S. manufacturing, which has suffered heavy job losses as a result of automation, trade deals, and other factors. In July, the Trump administration even celebrated “made in America” week by showcasing things built in the United States and…

Christopher Martin
Nearly two decades ago, rising from the ashes of the once-giant video game hardware manufacturer Sega, Microsoft debuted the Xbox and entered into the video game market with the intent of competing directly with Sony’s PlayStation brand. By 2006, Microsoft’s launch of the second-generation of Xbox…

Lily Elefteriadou
What self-driving cars want, and what people want from them, varies widely. Often these desires are at odds with each other. For instance, carmakers—and the designers of the software that will run autonomous vehicles—know that it’s safest if cars stay far away from each other. But traffic…

Dane Warren
Sponsored Content
As businesses become increasingly dependent on an effective supplier network, more data must be shared with these suppliers to support business goals and delivery business value. This gives rise to the need for a more robust, next-generation approach to supplier assurance and…

Ken Kingery
The first in-car measurements of exposure to pollutants that cause oxidative stress during rush-hour commutes has turned up potentially alarming results. The levels of some forms of harmful particulate matter inside car cabins was found to be twice as high as previously believed.
Most traffic…

Timothy Zimmerman
Cybersecurity, at this point in the technological age, has become a household word. Every week, almost like clockwork, it seems there is a story in the news about a newly discovered hack or data breach, often made possible by poor cybersecurity practices. Many of these incidents are focused around…

Chad Kymal
When Philip Crosby announced zero defects as a philosophy during the 1970s, it was met with incredulity. There were already many articles written on the fallacy of such a strategy and the enormous costs of moving toward zero defects. Fast forward 40+ years, and zero defects has become a reality.…

Therese Graff
Medical device companies use ISO 14971 to identify and manage user risks with their devices. However, we often find these same companies do not manage their project risks well.
What is project risk management?
The Guide to the Project Management Body of Knowledge (Project Management Institute,…

Ben Snedeker
Storms like Hurricanes Irma and Maria as well as other natural disasters bring with them lots of uncertainty: Where will they go? How much damage will they cause? What is certain is that no matter where they strike, natural disasters knock out power.
And no power means no internet for thousands…

Knowledge at Wharton
NASA Chief Astronaut Chris Cassidy has lived for months on the International Space Station and has performed six spacewalks. “Imagine hanging out with a glass bubble on your head, one hand on a hunk of metal, Earth going beneath your feet at five miles a second, and the whole world listening to…

Ann Cleland
If your hospital or clinic uses a Windows 7-based version of a Siemens PET/CT or SPECT system, it could be vulnerable to attack by a relatively low-skill hacker, according to a July 26, 2017, security advisory from the company.
The Industrial Control System Cyber Emergency Response Team (ICS-CERT…

Mike Richman
Our most recent episode of QDL from Friday, Sept. 29, 2017. featured news, technology, and two great interviews. Let’s have a closer look:
“Domestic Cars Fail to Keep Up With International Competition”
The most recent American Customer Satisfaction Index (ASCI) survey took a look at people’s…

Jack Phillips, Patti Phillips
According to W. Edwards Deming, “Every system is perfectly designed to achieve exactly the results it gets.” This applies to any quality initiative and any other activity, including learning and development. Yet, according to an ATD/ROI Institute study, only 8 percent of CEOs see the results most…

Anna Abram
We’re at a moment of extraordinary opportunity to improve public health. New innovations are giving us fundamentally better ways to address disease. Some of the same technology is providing consumers with a broader selection of foods that can improve peoples’ diets and products that can expand…

Arun Hariharan
In my October 2013 column, “Standardize to Improve,” I dealt with business process mapping in detail. Business process management systems (BPMS) comprise the entire gamut of documenting process steps, assigning ownership to process owners, and often, process-compliance audits to check whether you…

Mary McAtee
True to my profession as an engineer, I am a total geek at heart and proud of it. Spending time in automobile museums always fascinates me. It excites me to see a prescient innovator from the past come up with an idea like headlights. The first ones were Limelight carbide models that had a nasty…

Thomas Kochan, Lee Dyer
The technologies driving artificial intelligence (AI) are expanding exponentially, leading many technology experts and futurists to predict that machines will soon be doing many of the jobs that humans do today. Some even predict humans could lose control over their future.
While we agree about…