Sponsored Content
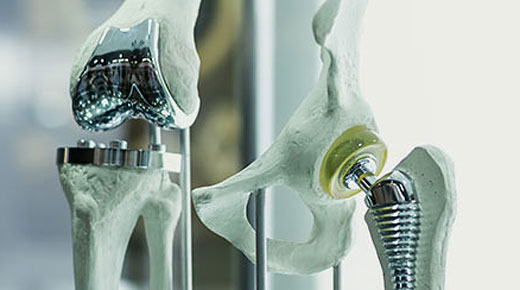
Founded in 1927 to produce aluminum splints—cutting edge at the time—Zimmer Biomet is a medical device company commanding second place in the entire world’s overall orthopedic market share. The organization’s stated purpose is to “Restore mobility, alleviate pain, and improve the quality of life for patients around the world.” For at least one Zimmer Biomet quality engineer, the nature of the company’s products and what it takes to manufacture them takes on a decidedly sensitive tone.
|
ADVERTISEMENT |
“For us, yes, we make great products, and yes, our company has a good stock price, but when you see a patient walking around with your product in them, it’s pretty compelling,” says Jeff Livingston, senior quality engineer at the Zimmer Biomet complex in Warsaw, Indiana. “I’ve done some pretty cool things in automotive, but what we do here is huge. Mostly it’s about regular folks just trying to live again, trying to get through life without pain.”

…
Add new comment