All Features

Jessica Reiner
For more than 20 years, a class of man-made, potentially cancer-causing chemicals called per- and polyfluoroalkyl substances (PFAS) has commonly been found in humans and the environment. These chemicals are used in a variety of industries and can be found in many consumer products, such as food…

Dirk Dusharme
Around the world, local agencies and institutions have scrambled to find personal protective equipment (PPE) to protect their essential employees from Covid-19. Not just healthcare workers, but also the men and women who to work to keep our cities and counties up and running, from emergency…

Stanley Chao
‘Can you help me source PPEs from China?” asks a caller on the phone. I have received dozens of these inquiries since March from local governments, medical clinics, and mom-and-pop shops after hospitals and first responders began reporting massive shortages of N95 masks, latex gloves, and surgical…

Quality Digest
It’s easy to assume that something as simple as a mask wouldn’t pose much of a risk. Essentially, it’s just a covering that goes over your nose and mouth.
But masks are more than just stitched-together cloth. Medical-grade masks use multiple layers of nonwoven material, usually polypropylene,…

Grant Ramaley
The International Accreditation Forum (IAF), the association of conformity assessment accreditation bodies worldwide, held an emergency meeting after confirming what appears to be an outbreak in the use of fake ISO 13485 certificates. ISO 13485 is a quality management system standard particular to…

Carrie Van Daele
Crossing the street or stepping backward when you encounter another person has already become a habit, as has a routine elbow bump, instead of a handshake.
And that is definitely what is needed during a health crisis. But when the time is right, as a society we must bounce back to social…

Gleb Tsipursky
So many companies are shifting their employees to working from home to address the Covid-19 coronavirus pandemic. Yet they’re not considering the potential quality disasters that can occur as a result of this transition.
An example of this is what one of my coaching clients experienced more than a…

Sangeet Paul Choudary
The digitization of patient data and the adoption of cloud-based healthcare management systems have created efficiencies and new business models across the value chain. Advancements in AI provide superior decision support systems to doctors, while connected devices enable the remote delivery of…

Jon Speer
Historically, risk management has been a complex and polarizing subject, with various stakeholders assigning different values on the probability and severity of harm. In the medical device industry, risk management’s high importance has led to the publication of standards such as ISO 14971,…

Alan Rudolph, Raymond Goodrich
We [Alan Rudolph and Raymond Goodrich] are both biotechnology researchers and are currently seeking to repurpose an existing medical manufacturing platform to quickly develop a vaccine candidate for Covid-19.
This process is used for the treatment of blood products such as plasma, platelets, and…

Amber Dance, Knowable Magazine
This story was originally published by Knowable Magazine.
As Covid-19 cases fill the hospitals, among the sickest and most likely to die are those whose bodies react in a signature, catastrophic way. Immune cells flood and attack the lungs they should be protecting. Blood vessels leak; the blood…
Jay Arthur—The KnowWare Man
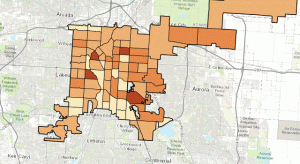
Story update 5/6/2020: The charts and some data have been updated to reflect the data available on the date this article was published.
During the Covid-19 stay-at-home order in Colorado, I've become increasingly frustrated by Covid-19 charts. Most of what I see are cumulative column charts, which…

Donald J. Wheeler, Al Pfadt
Each day we receive data that seek to quantify the Covid-19 pandemic. These daily values tell us how things have changed from yesterday, and give us the current totals, but they are difficult to understand simply because they are only a small piece of the puzzle. And like pieces of a puzzle, data…

William A. Levinson
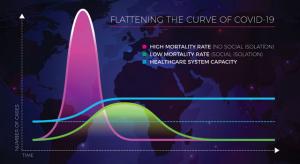
The phrase “flatten the curve” means to slow the transmission of the coronavirus (Covid-19) in order to spread the total number of cases out over a longer period of time. This will avoid overwhelming the healthcare system.1 The model is accurate as presented throughout the internet, but it also…
Clinton Ballew
Telehealth services have become even more critical in caring for patients as the Covid-19 pandemic quickly evolves. To temporarily remove barriers to practice telehealth, the federal government and many states have made sweeping changes in telehealth waiver provisions.
As HORNE continues to…

Eric Stoop
According to the National Safety Council, the rate of preventable workplace fatalities per 100,000 workers has flattened or risen slightly since 2009 after decades of steady improvement in occupational safety.
Companies conducting layered process audits (LPAs) can help get the United States get…
James Anderton
While healthcare professionals globally struggle to contain the Covid-19 pandemic, acute care patients are taxing ICU units worldwide. Critical to the care needed for the most serious cases is breathing support through mechanical ventilators. In Italy, the worst-hit nation in Europe, the lack of…
Donald J. Wheeler, Al Pfadt, Kathryn J. Whyte
This article is an update to “Tracking Covid-19” that Al Pfadt, Kathryn Whyte, and I wrote last week. In that article we summarized what is known about Covid-19, what has already happened, and what is to be expected based on the analysis of the data and the epidemiological models.
Over the past…

Ryan E. Day
Although Covid-19 shelter-in-home edicts usually use the terms “essential” and “nonessential,” most business owners think of doing business as essential for survival. Many organizations don’t have the resources to temporarily suspend business. They must find new ways to get it done.
In a Think…

Gleb Tsipursky
The Covid-19 coronavirus has developed into a widespread pandemic. With growing outbreaks of diagnosed cases in all 50 states, and vastly larger numbers of undiagnosed cases, there’s serious cause for concern. Yet quality professionals who follow the official advice on Covid-19 coronavirus prep…

David Pride
‘That escalated quickly!” is a common trope used in popular culture to describe when a situation gets out of hand before you’ve even had a chance to think about it. We don’t often use this trope in medicine, but I can think of nothing better to describe what has been going on in the United States…

Kevin Meyer
A couple weeks ago a consultant friend of mine, who coincidentally focuses his practice on lean in healthcare, was complaining about issues with his healthcare providers. It’s a story we hear often: doctors running late, very short and often superficial consultations, a rush to diagnosis, and a…

Sriram Chandrasekaran
Imagine you’re a fossil hunter. You spend months in the heat of Arizona digging up bones only to find that what you’ve uncovered is from a previously discovered dinosaur.
That’s how the search for antibiotics has panned out recently. The relatively few antibiotic hunters out there keep finding…
Sheng Lin-Gibson, Vijay Srinivasan
Biopharmaceuticals, also known as biological drugs or biologics, are manufactured from living organisms, or contain living organisms that have been genetically engineered to prevent or treat diseases. Biologics are chemically and structurally complex, and often highly heterogeneous; therefore,…

Peter Dizikes
Given the complexities of healthcare, do basic statistics used to rank hospitals really work well? A study co-authored by MIT economists indicates that some fundamental metrics do, in fact, provide real insight about hospital quality.
“The results suggest a substantial improvement in health if you…