All Features

ISO
There’s more than one path to service management. It refers to all the activities, policies, and processes that organizations use for deploying, managing, and improving IT service provision. In today’s technology-driven corporate landscape, the two leading methodologies come from the world of…
Adrian Hernandez, C. Michael White
T
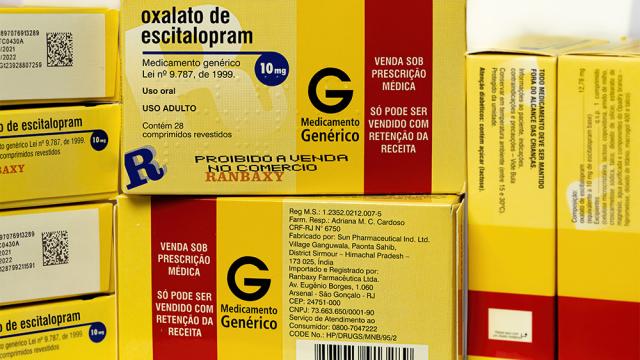
he U.S. Food and Drug Administration (FDA) regularly inspects manufacturing facilities to ensure that drugs meet rigorous quality standards. These standards are vital to protect patients from drugs that are incorrectly dosed, contaminated, or ineffective.
But over the past few years, tens of…

Nate Burke
How many of us remember walking into a holiday gift shop when younger (and before a global pandemic put a stop to the H word), and eagerly searching for a fridge magnet, mug, or pencil inscribed with our name?
Personalization is a tactic brands and businesses have been using for years to hook us…
Karla Raines
Total quality management (TQM) was in vogue during my undergraduate years and early career in industrial engineering. The United States was catching up to the Japanese in manufacturing production as their Toyota vehicles outperformed our Fords. A company couldn’t deliver a competitive product…
Harry Hertz
Each year after the Quest for Excellence Conference, I sift through my notes and try to identify themes I have heard in the presentations of the new Baldrige Award recipients. The most recent summary was after the 2019 conference (the 2021 conference included recipients from the last two years).…

Del Williams
To meet increasingly strict compliance standards, such as the Food Safety Modernization Act (FSMA) and Global Food Safety Initiative (GFSI), food processors now regularly use adenosine triphosphate (ATP) testing to monitor equipment surfaces for microbial growth. Add to this the need to minimize…

Nate Burke
It has been more than a year since retailers were forced to temporarily shut their doors or put in place restrictions to limit the in-store experience. Now, as we return to some semblance of normality, it’s essential that trust and brand value are retained for those operating a digital-only…

Chip Bell
We live in an era of statue removal. Meanwhile the largest mountain carving in the world is under construction in the Black Hills of South Dakota just 17 miles from Mount Rushmore. The final carving will be 640 feet long and more than 50 stories high. The subject of that carving? Crazy Horse.…

Gleb Tsipursky
When the Covid pandemic swept through the country last year, companies rapidly transitioned employees to working from home (WFH). However, this shift led to growing challenges of WFH burnout and Zoom fatigue.
Unfortunately, organizations treat these issues as day-to-day challenges, instead of…

Jon Speer
Demonstrating identification and traceability in all quality system processes is a must for medical device companies to comply with FDA regulations. To satisfy this compliance need, companies will need to connect related processes within their quality system to close the loop between related pre-…

Wade Schroeder
Medical-device usability testing and validation are critical tasks leading up to a medical device’s debut on the market. “Usability” looks at how the user interacts with your device and forms a key component of overall risk management and safety.
If there’s any “spoiler alert” to this article, it’…

Ariane Ollier-Malaterre, Knowable Magazine
This story was originally published by Knowable Magazine.
Working from home—formally known as telework—is here to stay. A 2021 survey of approximately 30,000 Americans concluded that, after the pandemic, 20 percent of all work days may continue to take place at home, vs. just 5 percent before.…

David L. Chandler
This story was originally published by MIT News.
Two MIT professors have proposed a new approach to estimating the risks of exposure to Covid-19 under different indoor settings. The guideline they developed suggests a limit for exposure time, based on the number of people, the size of the space,…

Jon Speer
The medical device technical file is a must-have document for devices to be sold in the European Union (EU) marketplace. The file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. It’s essentially an “everything you must…

Emily Newton
Food manufacturers must carry out numerous specific processes to check that the foods they produce and distribute are safe for consumers. Analytical testing plays a vital role in meeting that goal. Here’s a look at how such examinations raise food quality and purchaser trust.
Checking foods for…
Janet Woodcock, Judy McMeekin
During the past year, the U.S. Food and Drug Administration’s (FDA) approach to foreign and domestic inspections for food and medical products has been both risk-based and deliberate. The Covid-19 pandemic required us to rework our business operations so that we could carry out our public health…

Chip Bell
Iam often asked by customer service leaders how to get the CEO to care about customers. They are convinced there is a missed tactic that, if implemented, would have the C-suite camping out in the contact center and inviting customers to board meetings. When I outline a number of possible approaches…

Edmund Andrews
Seems everybody has a horror story about health insurance: Kafkaesque debates with robotic agents about what is and isn’t covered. Huge bills from a doctor you didn’t know was “out of network.” Reimbursements that take months to process.
It’s no secret that healthcare in the United States is…
Nate Burke
Unfortunately, a website is no longer enough for a significant or successful digital presence. Essentially, a presence is nonexistent without some consideration of search engine optimization (SEO).
But this, too, has become one of the basics of “going digital”—a must, rather than a “nice to have…

Graham Freeman
Here’s an unfortunate truth: The story of the Covid-19 pandemic is one of epic quality failures in almost every area imaginable. Although there have been some admirable successes, such as the food and beverage organizations that have ensured the continued safe delivery of food supplies to most…
Rita Men
Ending the pandemic depends on achieving herd immunity, estimated at 70 percent or even 80 percent to 90 percent of a population. With some 30 percent of Americans telling pollsters they have no interest in getting vaccinated, that’s cutting it a bit close. The numbers are even worse in many other…

Sharona Hoffman
Artificial intelligence holds great promise for improving human health by helping doctors make accurate diagnoses and treatment decisions. It can also lead to discrimination that can harm minorities, women, and economically disadvantaged people.
The question is, when healthcare algorithms…
Dave Klumpe
Recent surveys point to increasing frustration and, frankly, exhaustion among nurses across the country. Although attending to patients during the pandemic has exacerbated the challenges of the profession, nursing shortages have been reported on for well over a decade. It is incumbent on hospitals…

Annette Franz
The terms “customer-centric” and “customer-centricity” get thrown around a lot; oftentimes, it’s quite clear that they’re being used out of turn. I believe “customer-centric” is often confused with “customer focus,” but the two are very different.
Let’s look at some definitions.
Customer focus …
Tinglong Dai, Christopher Tang, Ho-Yin Mak
More than 50 million Americans have received at least one dose of either the Pfizer or Moderna Covid-19 vaccine. So far, Americans have been largely brand-agnostic, but that’s about to change as the Johnson & Johnson vaccine rolls out.
The vaccine has been hailed as a game changer. It requires…