All Features

Gleb Tsipursky
In an era when flexibility and autonomy are requirements, financial services leaders are ready to break the chains of traditional office norms. The results of a recent Deloitte and Workplace Intelligence survey make it clear: The future of the financial services sector is hanging in the balance,…
Scott A. Hindle, Douglas C. Fair
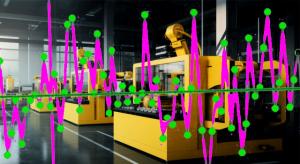
We are one year away from the 100th anniversary of the creation of the control chart: Walter Shewhart created the control chart in 1924 as an aid to Western Electric’s manufacturing operations. Since it’s almost prehistoric, is it now time to leave the control chart technique—that started out using…
Rob Moorey
Growing medical equipment inventories and increasing technical complexity are demanding more than ever from the clinical engineering teams responsible for maintaining clinical assets. Simultaneously, a shrinking talent pool of biomedical equipment technicians (BMETs) could lead to large staffing…

Stephanie Ojeda
In December 2023, the U.S. Food and Drug Administration (FDA) expects to issue its long-awaited overhaul of its Quality System Regulation (QSR). The biggest change is that the new Quality Management System Regulation (QMSR) will harmonize with ISO 13485 for medical device quality management. With…

Aymen Saidane
As the manufacturing world pushes toward the goal of zero-defect production, part inspection is critical. Sheet metal’s thinness, coupled with its susceptibility to warping, demands precision inspection—even the most minor deviations have significant implications down the line. This underscores the…
Steve Thompson
If you’ve ever enjoyed the experience of an audit or inspection, then you know it’s about as much fun as having your wisdom teeth extracted. As painful as audits and inspections may be, they are necessary to bring needed medical products to market and monitor them to protect consumers and patients…

Bryan Christiansen
Deciding whether to repair or replace an asset can be difficult. That’s why maintenance and reliability managers perform an analysis to determine whether it’s more economical to repair a failing asset or replace it with a new one. This process helps minimize total costs while ensuring that your…
Douglas C. Fair, Scott A. Hindle
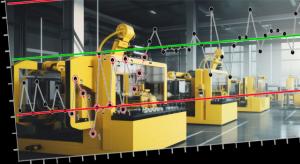
Today’s manufacturing systems have become more automated, data-driven, and sophisticated than ever before. Visit any modern shop floor and you’ll find a plethora of IT systems, HMIs, PLC data streams, machine controllers, engineering support, and other digital initiatives, all vying to improve…

Melissa Stewart
Companies are always looking for ways to bring in fresh ideas and new perspectives. And in an ever-evolving digital world, you can’t blame them. Young talents possess the latest technological skills and insights, which can be incredibly useful in adapting to the times. However, there’s one thing…

Stephanie Hinton
If you’re conducting a clinical investigation of a medical device in a European Union member state, you will be required to submit a clinical investigation report (CIR) along with a summary of the CIR to that member state.
The European Union Medical Device Regulation (EU MDR) lists this as one of…

Stephanie Ojeda
The number of ISO 45001 certificates is growing fast, jumping 54% from 2020 to 2021, according to the ISO Survey.
This occupational health and safety standard is especially prevalent in manufacturing, where managing safety incidents is a core concern from the perspective of protecting workers,…

William A. Levinson
The difference between common (or random) cause and special (or assignable) cause variation is the foundation of statistical process control (SPC). An SPC chart prevents tampering or overadjustment by assuming that the process is in control, i.e., special or assignable causes are absent unless a…

Peter Nathanial, David Zuluaga Martínez, Theodoros Evgeniou, Francois Candelon
Last month, the heads of seven major American AI companies emerged from the White House with an agreement on “self-regulation.” On the other side of the Atlantic, Europeans debate the long-awaited EU AI Act, the next major digital regulation following the EU’s Digital Services Act (DSA). The DSA is…

William A. Levinson
Inflation is a serious national issue. Credit agency Fitch Ratings just downgraded the U.S. credit rating—as in the “full faith and credit of the United States”—from AAA to AA+.1 This doubtlessly reflects the fact that our national debt exceeds $31 trillion, or almost $100,000 for every American,…

Stephanie Ojeda
Untitled Document
Workplace safety incidents are a key driver of risk in manufacturing organizations. There are the obvious risks to workers, whose ability to make a living directly depends on their employer’s approach to safety.
There are also huge risks to companies themselves, which face…

Mark Graban
I can’t count how many times during the past 20 years I’ve heard executives complain that their people aren’t enthusiastically participating in their lean program. Leaders lament that while the company has spent a small fortune to put everybody through continuous improvement training, hardly…

Andrey Koptelov
In this age of rapid technological innovation, the introduction of sophisticated technologies in various industries has raised complex ethical dilemmas. As businesses strive to achieve financial goals and keep stakeholders happy, they also have to mitigate the adverse effects of technology…

Matthew M. Lowe
Let’s start with a definition of Industry 4.0, keeping in mind that we’re rapidly approaching Industry 5.0. Industry 4.0 is an era marked by enhanced digitization and the increased connectivity of smart technologies. Where Industry 5.0 is more values-driven, it will require the technology of…

Jeffrey Lewis
I’ve observed that ISO management system audits have remained largely unchanged, even after the advent of ISO 19011:2018, the auditing standard that superseded ISO 19011:2011. Auditors are still using clause-based auditing, despite ISO 19011:2018’s direction to take a risk-based approach.…

Kurt Kleiner, Knowable Magazine
When a Manhattan parking garage collapsed in April 2023, rescuers were reluctant to stay in the damaged building, fearing further danger. So they used a combination of flying drones and a doglike walking robot to inspect the damage, look for survivors, and make sure the site was safe for human…

Emily Newton
Solving problems goes beyond noticing the symptoms and wanting to resolve them. It’s also necessary to perform a root cause analysis, pinpointing the factors likely to have made an issue occur. It’s only then that leaders can create concrete solutions for lasting changes. However, root cause…

Lindsey Walker
In the quickly changing industrial landscape, firms continue to place a high premium on safety. Innovative approaches to improving industrial safety have been made possible by technological advancements. One particularly revolutionary option is computerized maintenance management system (CMMS)…

Ujjwal Parwal
Risk analytics is a vital component of risk management that uses statistical models, data analysis, and predictive modeling techniques to assess, quantify, and mitigate risks in various domains. This article will delve into the definition of risk analytics, discuss its importance, and explore its…
Etienne Nichols
On Feb. 23, 2022, the U.S. Food and Drug Administration (FDA) released its proposed rule for the new Quality Management System Regulation (QMSR). The proposed QMSR will be the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s Quality System Regulation (QSR…

Michael Jarrett
History is filled with tales of courageous and decisive heroes. Individuals like Julius Caesar and Winston Churchill, for example, have led from the front to guide people through adversity and achieve ultimate success. This myth building is especially prominent in business, with stories of…