Scientists Look to New Technologies to Make Food Safer
Nearly every month, it seems, comes a new report. In March 2018, there was news of contaminated romaine lettuce, which eventually led to five deaths and sickened more than 200 people across the United States and Canada.
Identify, Protect, Detect, Respond, and Recover
The NIST Cybersecurity Framework consists of standards, guidelines, and best practices to manage cybersecurity-related risk. The framework’s prioritized, flexible, and cost-effective approach helps to promote the protection and resilience of critical infrastructure a
The Benefits of a Connected Quality Platform
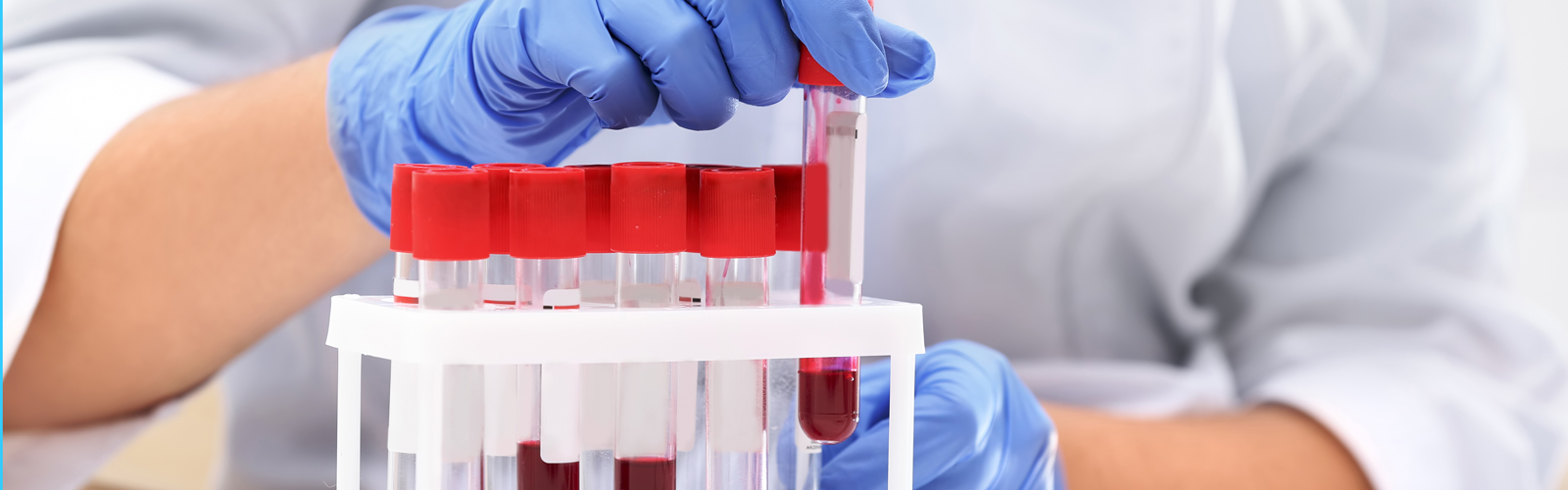
BioBridge Global (BBG) is a parent organization for four subsidiary organizations, three of which are involved in production activities, and they’re all around regenerative medicine, including blood components, clinical laboratory testing, and cell and tissue therapi
Canada's MDSAP Mandate Could Be Bad for Canadian Healthcare

The Dec. 31, 2018 deadline looms for medical device companies that sell their devices in Canada.
Life Science Pioneer Takes Cues From 21 CFR and ISO 13485
One of the unique aspects of Finch Therapeutics is that although its product does not fall easily into any regulated category and thus is not FDA-approved, the company has been working closely with the agency for at least five years.
Top Five Things Life Science Companies Need to Know about ISO and FDA Requirements

Life science companies play a major role in the global economy, with revenues expected to reach a staggering $1.5 trillion by 2020.1 Such a rosy forecast is likely to attract innovators and encourage current industry players to blaze new trails.
Use ISO 45001 to Support OSHA VPP Star Status
Chad Kymal1 gave an excellent overview of the ISO 45001 occupational health and safety (OHS) standard that was released in March 2018.
Inside Quality Digest Live for Nov. 2, 2018
Our industry embodies many aspects, but “Big Q” quality generally involves issues affecting management, measurement, and methodologies. This week on QDL, we covered all of them, and more. Let’s look closer:
Preparing for Quality
For manufacturers in diverse sectors such as automotive, aerospace, electronics, and medical device, there’s little question that ensuring great quality would be impossible without the proper testing of materials.
Pagination
- Previous page
- Page 63
- Next page