All Features
Ryan E. Day
One of the unique aspects of Finch Therapeutics is that although its product does not fall easily into any regulated category and thus is not FDA-approved, the company has been working closely with the agency for at least five years. The FDA has broad jurisdiction to regulate all health products,…

Matthew M. Lowe
Life science companies play a major role in the global economy, with revenues expected to reach a staggering $1.5 trillion by 2020.1 Such a rosy forecast is likely to attract innovators and encourage current industry players to blaze new trails. Whether new or established, life science companies…

The Un-Comfort Zone With Robert Wilson
Last year I was invited to give a lecture on critical thinking to the U.S. Navy. I opened my presentation with a story I’d read in Reader’s Digest magazine as a child. It’s an old story you may have heard before, but it’s a perfect introduction to the importance of critical thinking. Here’s how it…

Mike Richman
IMTS was a blast, but it was great to be back home in lovely Northern California this week. On this episode of QDL, we covered the skills that workers need and the innovations that organizations want. Plus, we brought you a live interview with author Mark Graban, and one on tape from Burt Mason of…
Rip Stauffer
I must admit, right up front, that this is not a totally unbiased review. I first became aware of Davis Balestracci in 1998, when I received the American Society for Quality (ASQ) Statistics Division Special Publication, Data “Sanity”: Statistical Thinking Applied to Everyday Data. At the time, I…
Bita Kash, Stephen L. Jones
Can you imagine a future where the question, “Did you bring a copy of your test results?” becomes entirely unnecessary? That could happen, but the methods that most healthcare providers use to exchange healthcare information are little different than they were 5,000 years ago, when physicians…

Knowledge at Wharton
‘How is it that in the middle of a relatively small town of about 125,000 people in Minnesota, you’ve got the No. 1-rated healthcare system probably in the world?”
The question was put to Jeffrey Bolton—the Mayo Clinic’s chief administrative officer—by Larry Jameson, executive vice president of…
Grant Ramaley
The Dental Trade Alliance learned from its members in February 2018 that the Canadian Health Ministry (“Health Canada”) had contacted the Standards Council of Canada (SCC) and the British Standards Institution (BSI). Health Canada had ordered these certification bodies to stop issuing ISO 13485…

Richard Pazdur
During the past decade, advances in understanding of cancer biology have led to the development of targeted treatments that are more effective than the chemotherapies of the past century. These therapies are demonstrating response rates large in magnitude or response durations prolonged in early…

Manfred Kets de Vries
A certain amount of stress is needed for us to function effectively. Stress is very much a part of the human condition. We all face disappointments, setbacks, losses and pain. But to live a rich and meaningful life, we must learn to deal in a constructive way with life’s challenges.
Stress evolved…

Sally Davies
It’s hard to believe that modern Western doctors, with their multimillion-dollar hospitals and high-tech gadgets, have much in common with their ancient counterparts. Up to the 19th century, doctors usually occupied a fairly low status in society. Doctors these days generally enjoy better working…
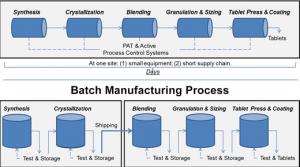
Scott Gottlieb
There’s new technology that can improve drug quality, address shortages of medicines, lower drug costs, and bring pharmaceutical manufacturing back to the United States. At the U.S. Food and Drug Administration (FDA), we’re focused on propelling these innovations, collectively referred to as…

Leonard L. Berry, D. Kirk Hamilton
We spend much of our time in buildings, and they can have a profound effect on our well-being, for better or for worse. As long ago as 1943, Winston Churchill told Britain’s House of Commons that “we shape our buildings, and afterwards our buildings shape us.”
Research is showing that effective…

Vanessa Burrows, Suzanne Junod, John Swann
During the early 20th century, Americans were inundated with ineffective and dangerous drugs, as well as adulterated and deceptively packaged foods.
A cosmetic eyelash and eyebrow dye called Lash Lure, for example, which promised women that it would help them “radiate personality,” in fact…
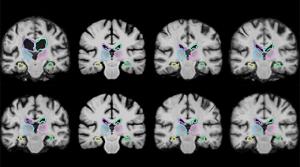
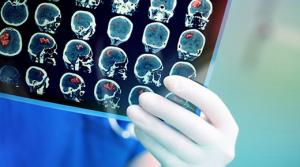
Rob Matheson
Medical image registration is a common technique that involves overlaying two images, such as magnetic resonance imaging (MRI) scans, to compare and analyze anatomical differences in great detail. If a patient has a brain tumor, for instance, doctors can overlap a brain scan from several months ago…

J. B. Silvers, Mark Votruba
The new healthcare venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase announced June 20, 2018, that Harvard professor and well-known author Atul Gawande would be the company’s CEO. The idea for the new company is to innovate by cutting costs from the healthcare system, starting with…
Sharona Hoffman
On June 12, 2018, the American Medical Association announced that drug shortages pose an urgent public health crisis. This crisis should be of concern to all Americans.
The Food and Drug Administration (FDA) defines a drug shortage as a “period of time when the demand or projected demand for a…

Janet Woodcock
The staff of the U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) always tries to utilize cutting-edge science and up-to-date process management, befitting our stature as the global “gold standard” in drug regulation. Maintaining that standard requires us to…

Mohammad Jalali
Like any large company, a modern hospital has hundreds, even thousands, of workers using countless computers, smartphones, and other electronic devices that are vulnerable to security breaches, data thefts, and ransomware attacks. But hospitals are unlike other companies in two important ways. They…

Bruce Hamilton
Last month I joined Eric Buhrens, CEO at Lean Enterprise Institute (LEI), to host a leadership team from Tel Aviv’s Sourasky Medical Center. They were on a study mission to many of Boston’s fine hospitals and were winding up their week with a visit to LEI. Early in the discussion, one of our guests…

Jon Speer
“I wish there was a way for the FDA to give me a heads-up about my stuff, prior to submission….”
That sentiment was really the basis behind the U.S. Food and Drug Administration’s (FDA) presubmission tool, as I was discussing recently with medical-device quality assurance and regulatory affiars…
Ryan E. Day
So, the Quality Digest team is considering a transition to working remotely for the most part. I and two other associates already do. In part one of this series, I outlined my ad-hoc attempt at creating a computer work space at home. The result was not very pretty.
As I said in part one, my home…

Marin Hedin
Limiting first-year medical residents to 16-hour work shifts, compared to “flexing” them to allow for some longer shifts, generally makes residents more satisfied with their training and work-life balance. It also makes their training directors more dissatisfied with curtailed educational…

Knowledge at Wharton
America’s healthcare system has been on the examining table lately: from the tortuous battle over the Affordable Care Act, to Senator Bernie Sanders’ bill to allow low-cost prescription drugs in from Canada, to the intriguing announcement in January that Amazon, Berkshire Hathaway, and JPMorgan…

Mike Richman
During this past Friday’s episode of Quality Digest Live, our weekly web TV show, QD editor in chief Dirk Dusharme and I covered stories about the gig economy and the skills gap and workforce shortages within manufacturing, especially as it relates to metrology, which is the science of measurement…