All Features

David Hall Rode
In 2025, there’s been a marked increase in FDA warning letters. During the second quarter of 2025 alone, the U.S. Food and Drug Administration (FDA) issued 172 warning letters. A notable enforcement surge occurred in September 2025 when the FDA released 80 warning letters in a single week. Although…

Stephen Russek
In the evenings, after patients have left for the day, our research team visits the radiation oncology offices at the University of Colorado Anschutz Medical Campus to talk to medical physicists about how our research can help cancer patients. We also run experiments in their radiation suites.
The…

Felicitas Stuebing
At the corner of quality and assembly, design engineers are frequently confronted with unexpected, complex fluid process issues in the prototyping phase. These obstacles are reflected in voice-of-customer sprints and surveys revealing that medical devices companies in particular stall out in the…

Elizabeth Weddle
The quality systems most medtech teams are stuck with aren’t built for how they work today. 21 CFR Part 820 was authorized by the Federal Food, Drug, and Cosmetic Act of 1978, long before the software industry even existed. And while the regulations themselves aren’t going anywhere, the world they…

Stephanie Ojeda
Implementing a new quality management system (QMS) is no small task, especially for life science companies faced with stringent regulatory requirements and a high validation burden. Entrenched legacy systems compound the problem as organizational inertia and complacency lead companies to make do…

Stephanie Ojeda
When organizations implement an enterprise quality management system (EQMS), the instinct is often to begin with high-visibility processes like corrective and preventive action (CAPA) or supplier quality. While these functions are critical, starting there can be a misstep. Without the right…

Walter Nowocin
Software selection, implementation, and ongoing maintenance are critical stages in the life cycle of biomedical software systems such as asset and calibration management platforms. Yet few industry resources provide detailed, practical guidance for managing these processes effectively.
One notable…

Frank King
At Ramirez & Co., a midsize business with decades of wins, leadership thought its biggest challenges were competitors, technology, and the market. Close, but no cigar. The real problem was stress, the silent drain that doesn’t show up on a Gantt chart but still wrecks your timeline.
Deadlines…

Judy Fainor
What if your quality system could detect and initiate corrective actions for equipment deviations before they affect product quality?
It’s a compelling vision—and one that’s becoming increasingly achievable through AI-enabled automation. But let’s be clear: We’re not there yet. What we do have is…

Jake Walton
Imagine you’re a student trying to pass a challenging class, one where the entire grade rests on the big test at the end of the semester. Fortunately, the professor handed out a syllabus that outlines exactly what will be on that final exam. Better still, you can also find a posted list of common…

Sachin Waikar
Take two aspirin and call me in the morning: If only prescribing medications were as simple as that.
In reality, the prescription process involves many players and steps. Details must be accurately spelled out, interpreted, and double-checked to ensure patients get the correct drug and dosage.
“…

Kamran Sayrafian
A few years ago, I heard on the news that many people were being hospitalized with a condition of excess fluid in the lungs, called pulmonary edema. It’s common in elderly patients. Pulmonary edema is dangerous and can lead to breathing difficulties and lung failure. Because it has the potential to…

Paul Hanaphy
The dental industry is seeing a surge in 3D printing, with the technology enabling a growing number of dentists to rapidly create custom implants in clinics around the world.
When it comes to customizing implants like dental crowns, bridges, guides, and aligners, 3D printing is faster, more…
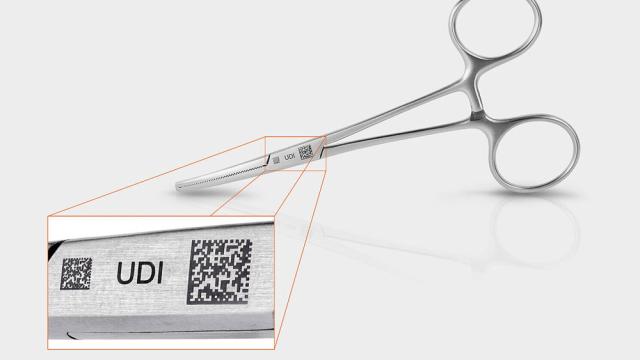
FOBA
In medical technology, safety is paramount. Every step in the manufacturing process must meet the highest standards, from patient protection to regulatory compliance. Because direct part marking plays a key role in ensuring traceability and unique device identification (UDI) conformity, it’s…

NIST
A rapidly growing category of drugs called protein-based biotherapeutics can be used to treat cancers and genetic and autoimmune disorders. These drugs, which usually take the form of large protein molecules, are manufactured by growing living cells that are genetically engineered to produce the…
Nimax
Pharmaceutical serialization practices are on the rise and have progressively become a worldwide standard as a result of stringent regulations in various of markets, including the United States, European Union, China, and Argentina. Recent estimations found that by 2022 serialization practices had…

Sam Schaffter
My grandfather was a carpenter, so growing up I often heard the adage, “Be sure to measure twice so you only have to cut once.” The saying is also attributed to tailors—you have to make sure your measurements are correct before you cut the fabric.
Little did I know that years later the saying…

Jennifer King
Although patient safety is paramount in healthcare settings, about 1 in 10 patients is harmed in healthcare, and more than 3 million deaths occur due to unsafe care, says the World Health Organization (WHO). The reality is hospitals and healthcare facilities face numerous challenges in managing…

Etienne Nichols
Good supplier management is one of the most important methods of building a safe and effective medical device. A single device may be made up of dozens of parts and components coming from several different suppliers, and many medical device companies outsource the manufacturing of their device to a…

ISO
The digital revolution has transformed healthcare along with virtually every other industry. From telemedicine to digital health data, providers now have access to innovative solutions that have the potential to make healthcare more accessible and effective for all.
In some instances, this is done…

Knowledge at Wharton
Many countries face the reality of demographic aging: Fertility is plummeting and people are living longer. This raises critical challenges for the labor market, healthcare, and long-term care markets, as well as retirement systems and financial planning. A Wharton symposium on the implications of…

Amy Knue
Health systems across the country are unknowingly paying multiple times for the same medical equipment—once to own it, and again to rent it. The issue isn’t always an increase in clinical demand; it’s often availability and visibility to medical device inventory. The cost of these unnecessary…

Etienne Nichols
The corrective and preventive action (CAPA) process is one of the most important elements within a medtech company’s quality management system (QMS). The goal of the CAPA system is to identify, address, and prevent systemic issues that could compromise product safety, regulatory compliance, and the…

Stephanie Ojeda
Many companies are still clinging to paper-based and unconnected electronic processes, despite the clear disadvantages. Without modern tools like QMS software, these organizations risk compromising product quality, falling behind in compliance, and ultimately losing competitive ground.
In contrast…
Etienne Nichols
As part of its effort to address the changing landscape around artificial intelligence (AI) in medical devices, the U.S. Food and Drug Administration (FDA) has recently released two new guidance documents on artificial intelligence-enabled device software functions (AI-DSF): • “AI-enabled device…