All Features

ISO
Healthcare administrators find themselves at the fore of a demanding and transformative field, where the pursuit of excellence in patient care is nonnegotiable. In a health industry landscape facing evolving regulations, escalating costs, and an increasing emphasis on patient outcomes, the need for…

Bryan Christiansen
Deciding whether to repair or replace an asset can be difficult. That’s why maintenance and reliability managers perform an analysis to determine whether it’s more economical to repair a failing asset or replace it with a new one. This process helps minimize total costs while ensuring that your…
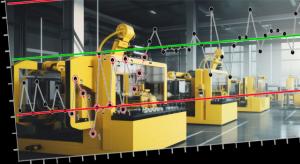
Douglas C. Fair, Scott A. Hindle
Today’s manufacturing systems have become more automated, data-driven, and sophisticated than ever before. Visit any modern shop floor and you’ll find a plethora of IT systems, HMIs, PLC data streams, machine controllers, engineering support, and other digital initiatives, all vying to improve…

Melissa Stewart
Companies are always looking for ways to bring in fresh ideas and new perspectives. And in an ever-evolving digital world, you can’t blame them. Young talents possess the latest technological skills and insights, which can be incredibly useful in adapting to the times. However, there’s one thing…

Wael William Diab
Artificial intelligence (AI) is everywhere—and that’s something to marvel at. AI is powering everything from advanced web searches to social media recommendations and video game design. But it could do infinitely more.
AI has the potential to revolutionize our societies and economies. Discussions…

James Chan
Implementing a computerized maintenance management system (CMMS) isn’t just a monetary investment. If you want to see real results, you’ll need to put in the effort to make sure the system is properly implemented and adopted. It isn’t as simple as flipping a switch.
It takes planning, time, and…

Stephanie Hinton
If you’re conducting a clinical investigation of a medical device in a European Union member state, you will be required to submit a clinical investigation report (CIR) along with a summary of the CIR to that member state.
The European Union Medical Device Regulation (EU MDR) lists this as one of…

Stephanie Ojeda
The number of ISO 45001 certificates is growing fast, jumping 54% from 2020 to 2021, according to the ISO Survey.
This occupational health and safety standard is especially prevalent in manufacturing, where managing safety incidents is a core concern from the perspective of protecting workers,…

William A. Levinson
The difference between common (or random) cause and special (or assignable) cause variation is the foundation of statistical process control (SPC). An SPC chart prevents tampering or overadjustment by assuming that the process is in control, i.e., special or assignable causes are absent unless a…

Peter Nathanial, David Zuluaga Martínez, Theodoros Evgeniou, Francois Candelon
Last month, the heads of seven major American AI companies emerged from the White House with an agreement on “self-regulation.” On the other side of the Atlantic, Europeans debate the long-awaited EU AI Act, the next major digital regulation following the EU’s Digital Services Act (DSA). The DSA is…

William A. Levinson
Inflation is a serious national issue. Credit agency Fitch Ratings just downgraded the U.S. credit rating—as in the “full faith and credit of the United States”—from AAA to AA+.1 This doubtlessly reflects the fact that our national debt exceeds $31 trillion, or almost $100,000 for every American,…

Stephanie Ojeda
Untitled Document
Workplace safety incidents are a key driver of risk in manufacturing organizations. There are the obvious risks to workers, whose ability to make a living directly depends on their employer’s approach to safety.
There are also huge risks to companies themselves, which face…

Mark Graban
I can’t count how many times during the past 20 years I’ve heard executives complain that their people aren’t enthusiastically participating in their lean program. Leaders lament that while the company has spent a small fortune to put everybody through continuous improvement training, hardly…

Kobi Leins, ISO
Untitled Document
In everyday life, the most common conversation about artificial intelligence (AI) goes along the lines of, “I used ChatGPT, and it did x.” Corporate leaders, governments, and international organizations, however, are having a very different conversation. Theirs is about how the…

Andrey Koptelov
In this age of rapid technological innovation, the introduction of sophisticated technologies in various industries has raised complex ethical dilemmas. As businesses strive to achieve financial goals and keep stakeholders happy, they also have to mitigate the adverse effects of technology…

Matthew M. Lowe
Let’s start with a definition of Industry 4.0, keeping in mind that we’re rapidly approaching Industry 5.0. Industry 4.0 is an era marked by enhanced digitization and the increased connectivity of smart technologies. Where Industry 5.0 is more values-driven, it will require the technology of…

engineering.com
In the era of the industrial internet of things (IIoT), assets of both information technology (IT) and operational technology (OT) are becoming more sophisticated—and they both generate and use more data. As a result, it’s increasingly important for manufacturers to mesh the IT and OT sides of…

Jeffrey Lewis
I’ve observed that ISO management system audits have remained largely unchanged, even after the advent of ISO 19011:2018, the auditing standard that superseded ISO 19011:2011. Auditors are still using clause-based auditing, despite ISO 19011:2018’s direction to take a risk-based approach.…

Kurt Kleiner, Knowable Magazine
When a Manhattan parking garage collapsed in April 2023, rescuers were reluctant to stay in the damaged building, fearing further danger. So they used a combination of flying drones and a doglike walking robot to inspect the damage, look for survivors, and make sure the site was safe for human…

Emily Newton
Solving problems goes beyond noticing the symptoms and wanting to resolve them. It’s also necessary to perform a root cause analysis, pinpointing the factors likely to have made an issue occur. It’s only then that leaders can create concrete solutions for lasting changes. However, root cause…

Lindsey Walker
In the quickly changing industrial landscape, firms continue to place a high premium on safety. Innovative approaches to improving industrial safety have been made possible by technological advancements. One particularly revolutionary option is computerized maintenance management system (CMMS)…

Ujjwal Parwal
Risk analytics is a vital component of risk management that uses statistical models, data analysis, and predictive modeling techniques to assess, quantify, and mitigate risks in various domains. This article will delve into the definition of risk analytics, discuss its importance, and explore its…
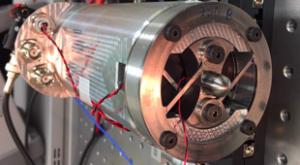
NIST
Static force, such as the weight of a person standing motionless on a bathroom scale or the force that an office full of equipment exerts on a high-rise floor, can be easily determined using scales, balances, load cells, and the like because static force doesn’t change over time. It’s…
Etienne Nichols
On Feb. 23, 2022, the U.S. Food and Drug Administration (FDA) released its proposed rule for the new Quality Management System Regulation (QMSR). The proposed QMSR will be the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s Quality System Regulation (QSR…

Michael Jarrett
History is filled with tales of courageous and decisive heroes. Individuals like Julius Caesar and Winston Churchill, for example, have led from the front to guide people through adversity and achieve ultimate success. This myth building is especially prominent in business, with stories of…