All Features

ISO
Cybersecurity has become increasingly critical in the digital age as organizations across all sectors face growing threats from cybercriminals.
Imagine that hackers breached a small healthcare practice through “phishing”—sending a scam email and gaining access to sensitive patient data, including…

Julie Gowthorpe
Management goals commonly include maintaining harmonious work environments that make employees happy and motivated. But how do you achieve this when people don’t get along? “Sort it out on your own” is no longer an option when employees are in conflict. As teams grow and experience turnover, a…

ISO
ISO and the United Nations Development Program (UNDP) have unveiled the world’s first international guidelines to help businesses and organizations expedite their contributions to the U.N.’s sustainable development goals (SDGs).
New guidelines for urgent action
The ISO/UNDP guidelines for…

Thierry Mantel
Metal cold-rolling is a critical process in manufacturing various steel and aluminum products. As manufacturers look for ways to modernize and streamline their manufacturing and quality control processes, digital technologies are poised to transform the metal cold-rolling industry by offering…

Stephanie Ojeda
How can a quality manager secure stakeholder engagement and hold individuals accountable for the quality of their work throughout the corrective and preventive action (CAPA) cycle?
With the burden of quality and compliance on their shoulders, it can be tempting for a quality manager to gather…

Seb Murray
Last year, the corporate world adopted a new term: flattening. This refers to how tech companies, which rapidly hired droves of middle managers during the pandemic boom, are now eliminating this layer through widespread job cuts.
Recent research by Mustafa Dogan, Alexandre Jacquillat, and Wharton’…
James Chan
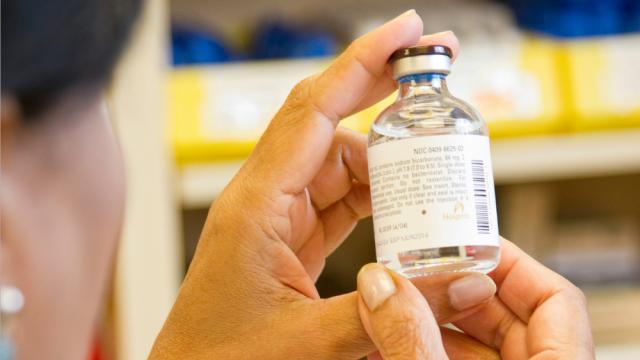
The U.S. Food and Drug Administration (FDA) is the country’s chief agency for regulating the manufacture, marketing, and distribution of critical consumer goods including food, cosmetics, medical devices, biological products, and pharmaceuticals. The FDA provides direct oversight of the businesses…

Nadav Klein
When conflicts in a team, no matter how minor, are left unresolved, they can eventually breed resentment. If unaddressed, this could lead to cynicism and distrust, as well as harm to individual and team performance. How should leaders deal with this?
The intuitive answer might involve…
Jennifer King
Many people don’t realize just how long AI has been around in the healthcare industry—and are surprised to find out that it’s something that’s been relied on for 50 years already.
MYCIN, a computer-based model with machine learning capabilities, was developed by a team of researchers at Stanford…

Michael Platt, Vera Ludwig
Four years after the Covid-19 pandemic accelerated remote work, its advantages and drawbacks have been well documented. For leaders, the biggest hurdles have remained constant: building employee engagement, trust, and communication.
Nano Tool
Scientists from the Wharton Neuroscience Initiative …

Robyn Coward, Brian Brooks
This year, the Medical Device Innovation Consortium held an Excellence in Quality Summit where it was promised that participants would receive a “unique opportunity to learn adoptable cutting-edge practices to maximize the impact of investing in quality across [their] total product life cycle.”…

John Tschohl
Are your employees empowered to make decisions on the spot in favor of the customer? Your single goal should be to have overly happy customers. Too many things go wrong each day. You want your employees to understand they are in customer service, and their No. 1 responsibility is to take care of…

Touradj Ebrahimi
For the last 30 years, the JPEG image format has been a staple for the internet’s billions of users. While the technologies used to display images have evolved tremendously during the past few decades, the JPEG format is still used everywhere. This is a great example of what can happen when a new…

John Hilgendorf
Whether you’re an executive with limited energy or an hourly employee trying to minimize work, the bottleneck in your productivity isn’t time or money but mental effort. And in a digital age where all data can be stored electronically, the most valuable functions of software—especially those in…

Mike Figliuolo
Reading the news (or even your email) can be distressing to the point of despondency. It can also be fun. It’s especially fun when people say or write silly stuff, and the reporter or editor has to write [sic] after a misspelling or a stupid comment in the original transcript. Sic, usually placed…

Etienne Nichols
On Jan. 31, 2024, the U.S. Food and Drug Administration (FDA) released its final rule for the new Quality Management System Regulation (QMSR).
The new QMSR is the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s quality system regulation (QSR) with the…
Stephanie Ojeda
There’s no question about it: Should an auditor or inspector visit your facility, one thing that will certainly be under the microscope is your corrective and preventive action (CAPA) system.
CAPA management is a recurring theme in U.S. Food and Drug Administration (FDA) warning letters, a fact…

George Schuetz
In this job, I get a lot of questions. In fact, I did some figuring the other day and estimated, conservatively, that we have probably answered at least 50,000 gauging questions over the past 35 years or so.
Some of these questions have been challenging. They’ve pushed me to learn more about my…
Chris Rush
Ensuring the accuracy and security of clinical data, as well as compliance with good clinical practice (GCP), will in large part determine the success of your study and regulatory submission to the U.S. Food and Drug Administration (FDA). Data management and reporting are essential practices when…

ISO
Global awareness of climate change and sustainability has grown exponentially during the past decade, making terms like corporate responsibility, carbon footprint, and transparency the new buzzwords of our time.
The drive toward a more sustainable future has propelled environmental, social, and…

Chip Bell
The cost of acquiring a new customer can be five to 10 times the cost of keeping an existing customer, depending on the industry. You already knew that. But while most organizations know why their customers opened the exit door, they devote little effort to understanding what prompted customers to…

Patty Davidson
For the U.S. Department of Labor’s Wage and Hour Division, protecting children in the workplace is the top priority. The division is responsible for enforcing foundational labor standards, including the child labor provisions of the Fair Labor Standards Act (FLSA). The FLSA establishes minimum wage…

James Chan
Maintenance teams operate in challenging environments that can often produce workplace mishaps, near misses, and other potential hazards. That’s where maintenance incident reports come in. Not only do these reports help team members isolate and problem-solve safety concerns, leading to a safer…

Luk Van Wassenhove
In today’s business environment, where the insatiable desire to grow profits overshadows environmental and social interests, it’s easy to overlook how the actions of economic actors can shape corporate—and environmental—outcomes.
In the accounting profession, practitioners exercise skepticism…

Stephanie Ojeda
Customer complaints are a fact of life in any industry. Even though manufacturers would prefer not to receive complaints, they do come with a silver lining. Managed effectively, they play an important role in improving your products and processes over time—that is, for companies that take a…