New Software Includes CFR Part 11 Compliance Features
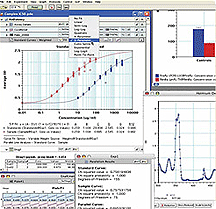 Molecular Devices Corp.
Molecular Devices Corp. Molecular Devices Corp.
Molecular Devices Corp. Six leading technology companies recently agreed to participate in a new program that will help create SAFE-BioPharma–enabled business applications, inf
Six leading technology companies recently agreed to participate in a new program that will help create SAFE-BioPharma–enabled business applications, inf A pharmaceutical researcher at Vanderbilt University Medical Center recently received an award from the American Society of Health-System Pharmacists
A pharmaceutical researcher at Vanderbilt University Medical Center recently received an award from the American Society of Health-System Pharmacists The Center for Professional Innovation and Education will host two upcoming conferences on effective document management for the pharmaceutica
The Center for Professional Innovation and Education will host two upcoming conferences on effective document management for the pharmaceutica CeTaQ Americas recently started offering machine-capability analysis testing for lasers used in medical and biomedical device manufacturing.
CeTaQ Americas recently started offering machine-capability analysis testing for lasers used in medical and biomedical device manufacturing. The National Standards Authority of Ireland will host a three-day course examining the new hazardous-substance–free specification.
The National Standards Authority of Ireland will host a three-day course examining the new hazardous-substance–free specification. Analyst firm DMG Consulting recently named NICE Systems as the market leader in contact call center quality management and liability recording.
Analyst firm DMG Consulting recently named NICE Systems as the market leader in contact call center quality management and liability recording. Despite numerous technological advances and tens of billions of investment dollars that were poured into information technology, a majority
Despite numerous technological advances and tens of billions of investment dollars that were poured into information technology, a majority Quality defects in new home construction could be prevented with routine quality assurance techniques, according to a recent survey.
Quality defects in new home construction could be prevented with routine quality assurance techniques, according to a recent survey. QC Inspection Services Inc.
QC Inspection Services Inc.© 2025 Quality Digest. Copyright on content held by Quality Digest or by individual authors. Contact Quality Digest for reprint information.
“Quality Digest" is a trademark owned by Quality Circle Institute Inc.