Kevin Coughlin / Brookhaven National Laboratory
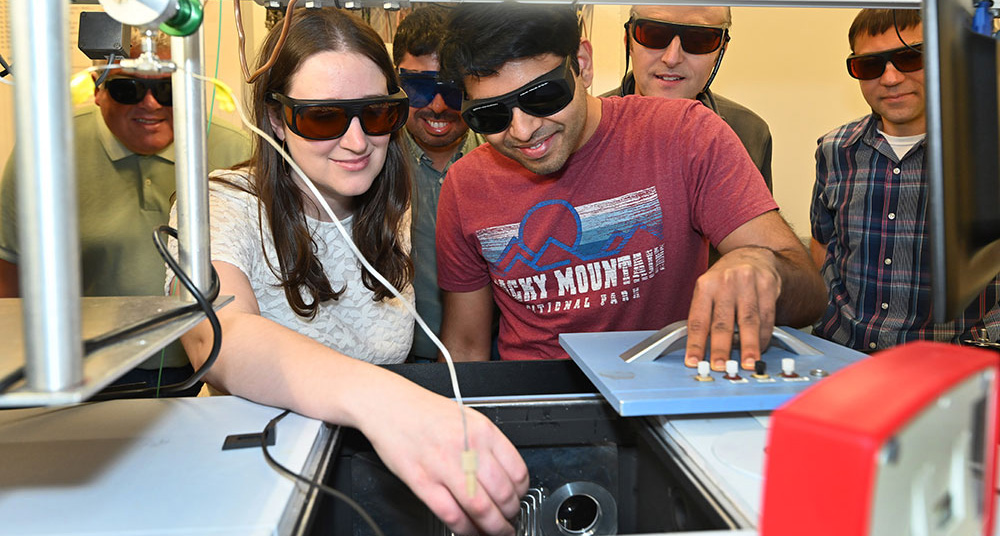
Andressa Müller and Sai Puneet Desai use an infrared spectrometer in the Brookhaven Lab Chemistry Division to analyze a sample containing a photosensitizer, their catalyst, and other reaction components. They and the rest of the research team wear protective glasses to shield their eyes from the laser that helps them understand the reaction mechanism.
Chemists at the U.S. Department of Energy’s Brookhaven National Laboratory have designed a new way to convert abundant carbon dioxide (CO2) into formate (HCO2-), an industrial chemical used as a fuel, as an antibacterial/antifungal agent, and for making pharmaceuticals. Their reaction uses a light-activated metal-centered catalyst to facilitate the transfer of electrons and protons needed for the chemical conversion.
|
ADVERTISEMENT |
“We are taking something cheap and abundant like CO2 and adding electrons and protons to convert it into something useful,” says Sai Puneet Desai, the lead author of a paper describing the research, just published in the Journal of the American Chemical Society.
In some ways, the process mimics photosynthesis, the series of reactions plants use to convert CO2 and water into sugar, their primary source of fuel. “In both our reaction and photosynthesis, the transfer of protons and electrons is promoted directly or indirectly by light,” Desai says.
“You can think of it as storing light energy in the chemical bonds,” says co-author Andressa Müller.
…
Add new comment