All Features

Pavel Kireyev
Good salespersonship is a species of street smarts. It’s about quickly sizing up your customers and pitching your wares in terms that reverberate with their unspoken needs and desires. As artificial intelligence (AI) and machine learning increasingly intersect with e-commerce, these priceless human…

Steven Brand
The food industry is evolving rapidly, with consumers demanding quality, authenticity, and transparency from food manufacturers. And they’re not just demanding it; they’re “voting with their dollars,” supporting companies that align with their personal beliefs. To keep up with consumer demand—and…

Jim Benson
Editor’s note: Read episode two in the Respect for People series here.
I was standing in a back room of the Honolulu Museum of Art that was off limits to the public. In this one room, protected from bugs, humidity, and light, was the world’s largest collection of Japanese woodblock prints. (My…

Annette Franz
I write about organizational culture and core values quite often. One of my most recent articles on this topic was about whether employees believe in their companies’ core values. I shared this statistic from Gallup: Only 23 percent of U.S. employees believe that they can apply the core values to…

Daniel Hess
We all expect hospitals to be open and operating when we need them, but extreme weather events like hurricanes are a strain on resources and pose significant challenges for hospitals.
Closing a hospital is an extreme action, but several hospitals in Florida, Georgia, and South Carolina did just…

Sharona Hoffman
A career as a physician has traditionally been considered to be among the best vocations that talented students can pursue. That may no longer be the case. All too many doctors report that they are unhappy, frustrated, and even prepared to leave the profession.
That should worry all of us. The…

Søren Block Olsen
Manufacturers face constant challenges of rising expectations as customers and regulators demand better quality and greater traceability throughout the supply chain. Exacerbating matters are unpredictable tariffs, which necessitate faster responses to changing trade barriers and regulatory…

Peter Rose
On May 26, 2020, the new European Union Medical Device Regulation (MDR) will finally take effect. By that date, all Class I manufacturers wishing to continue their trading activities within the EU market must have effectively completed the transition from the previous medical device directive and…

Annette Franz
Being a customer experience (CX) professional is hard enough; misinformation just makes our work more challenging. Misinformation or confusing information by a person with a ton of followers and a ton of influence makes our work even more challenging.
Recently, Seth Godin published a post on his…

Stephen McCarthy
In our constantly evolving, data-rich universe, collecting, interpreting, and understanding process data can be tricky. But it is increasingly important if we want to maintain sustainable quality across product development and manufacturing processes. This challenge is particularly evident in the…

Jennifer Lopez
Globalization of the medical device market as well as its supporting supply chains continues to increase year after year. This has forced regulatory bodies to grapple with finding a way to narrow the gap between international and domestic regulation. In spring 2018 the United States Food and Drug…

Michael D. Williams
As I spoke recently with colleagues at a conference in Florence, Italy, about healthcare innovation, a fundamental truth resurfaced in my mind: the U.S. healthcare industry is just that. An industry, an economic force, Big Business. It is a vehicle for returns on investment first and the success of…

Annette Franz
You’re listening to customers. You’re combining their feedback with those bread crumbs of data that they leave with every transaction and interaction with your brand. You’ve developed customer personas to better understand who they are, what problems they are trying to solve, and what jobs they…

Barnaby Lewis
Put in the terms of this article’s title, most of us would run a mile, whatever the proposition. But the popularity of online reviews, and the trust we place in persons unknown when making major decisions about where to stay, what to eat, and how to get the most from a trip, tells a different story…
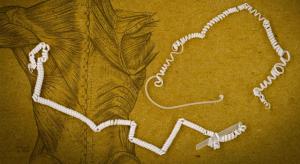
David L. Chandler
As a cucumber plant grows, it sprouts tightly coiled tendrils that seek out supports to pull the plant upward. This ensures the plant receives as much sunlight exposure as possible. Now, researchers at MIT have found a way to imitate this coiling-and-pulling mechanism to produce contracting fibers…
The FDA has announced an end to the alternative summary reporting (ASR) program for medical device manufacturers and will make the data publicly accessible.
The ASR program originally launched in 2000 when device manufacturers sought an “alternative summary” reporting exemption. ASR permitted…

Knowledge at Wharton
A recent family biking vacation in the Dolomites region of Italy had my family and I all swept up in the charms of Northern Italy. Snow-capped peaks near the Austrian border, endless apple orchards, award-winning Chenin Blanc, and quaint Italian villages with healthy doses of affogato (strong…

Annette Franz
There’s a lot of bad press out there about journey mapping. And there’s a lot of bad journey mapping (or what people think is journey mapping). A few months ago, I shared my six-step journey mapping process. Remember, journey mapping isn’t just a tool; it’s also a process. Know the tool and create…
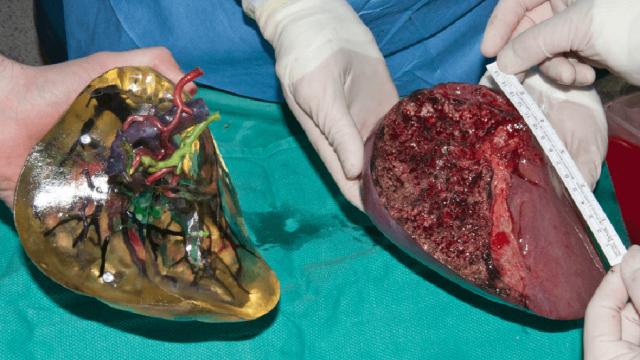
Sarah Webb, Knowable Magazine
This story was originally published by Knowable Magazine.
What you see in the image above is a lobe of a liver, times two. On the right, a flesh-and-blood one, removed from a transplant donor; and on the left, one created from plastic to represent bile ducts, arteries, and veins, which were laid…

Grant Ramaley
Although the “new approach” to regulating medical devices has always given more urgency to higher-risk medical devices, this is not the case for the European Medical Device Regulation (MDR). Class 1 medical devices must fully comply with the regulation by May 26, 2020, or be shut out of the region…

Jon Speer
This notion of risk-based processes within quality systems is something that has become part of our formal lexicon following the release of ISO 13485:2016, the globally harmonized standard for medical device quality management systems (QMS).
Well before these risk-based processes became a quality…

Matthew M. Lowe
While most business sectors have welcomed the efficiencies and benefits that cloud technologies and software-as-a-service (SaaS) offerings bring, the life sciences industry has been slow to embrace external cloud networks. Merely a decade ago, in fact, an International Data Corp. survey showed that…

David Dubois
Faced with a growing range of tech solutions in marketing, from AI to big data to blockchain, business-to-business (B2B) companies too often choose the status quo. Recent evidence suggests the divide between success and failure is not about how much companies spend, but how well they integrate…
Jon Speer
The European Medical Device Regulation (MDR) is a new set of regulations that governs the production and distribution of medical devices in Europe, and compliance with the regulation is mandatory for medical device companies that want to sell their products in the European marketplace.
If your…

Alex Bekker
Do you know what a retailer and a tightrope walker have in common? They both have to balance. For the tightrope walker, the logic is clear. But what’s the balance that a retailer is looking for?
A typical dilemma of shortages vs. storage costs
Although the dilemma of shortages vs. storage costs is…