Quality by design (QbD) is a widely discussed approach that is often neither well understood nor effectively executed. Joseph Juran also called QbD “quality planning.” QbD is not primarily focused on typical quality issues such as corrective action, testing, measurement, or monitoring. QbD is a holistic approach to planning to ensure quality is inherent in products and processes.
|
ADVERTISEMENT |
QbD starts with quality goals, and identifying customers and their true needs. This sets up the target and meaningful goals for the quality program. Meaningful goals are those that customers care about and will thus pay for in your products.
Product and process design drive quality performance. These are clearly shown on the inner ring in the U.S. Food and Drug Administration’s (FDA) QbD graphic in figure 1.
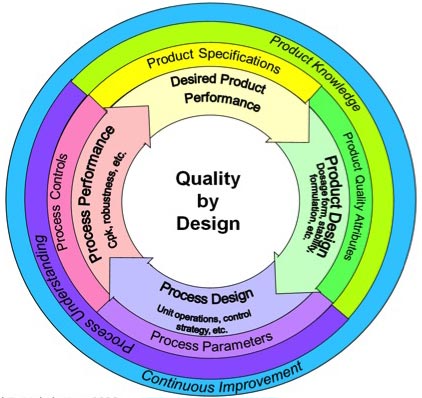
Figure 1: Quality by design (QbD) concept as presented by U.S. Food and Drug Administration (FDA)
…
Comments
Quality Planning Underutilized
Julie has hit on some excellent points. Quality Planning is one of 3 components of the Juran Trilogy. Many organizations are investing heavily in Quality Control, and even more so in Quality Improvement, but fail to take the lessons learned and apply to Quality Planning (a.k.a. Quality by Design). Therefore many products and services are "dead on arrival" because they are not designed to meet the current needs of customers, or the delivery processes are not designed to meet the required capability. Companies would be wise to re-balance their investment in Quality to address all 3 components of the Juran Trilogy. Their bottom lines will benefit, and their customers will benefit.
Add new comment