Thermo Fisher Scientific
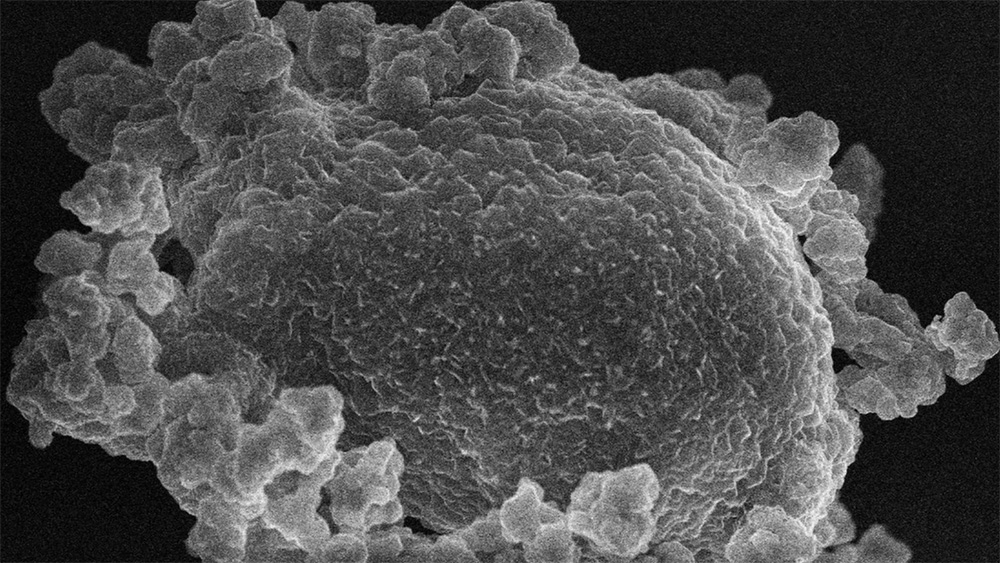
A scanning electron microscope captures the moments before lithium oxide (larger, middle particle) sublimates and mixes with the nickel-rich material surrounding it.
A common lithium salt has revealed new possibilities for manufacturing cheaper, longer-lasting battery materials.
|
ADVERTISEMENT |
The discovery centers on sublimation, a commonly known process whereby, under the right conditions, a solid turns directly into a vapor. Sublimation is what creates the tail of a comet as it flies by the sun. As the comet’s icy shell heats up, the ice instantly becomes vapor instead of first melting into liquid water.
Now, scientists at the U.S. Department of Energy’s Pacific Northwest National Laboratory (PNNL) have taken a page out of nature’s playbook. In a new finding published March 6, 2025, in Nature Energy, the PNNL-led team showed that vapor from lithium oxide (Li2O) sublimation accelerates a chemical reaction that forms single crystals when mixed with nickel-rich precursors. What’s more, the sublimation happens at just one atmosphere of pressure—the everyday pressure felt at sea level. Single-crystal battery materials are thought to help batteries last longer.
…
Add new comment