Lohyun Kim
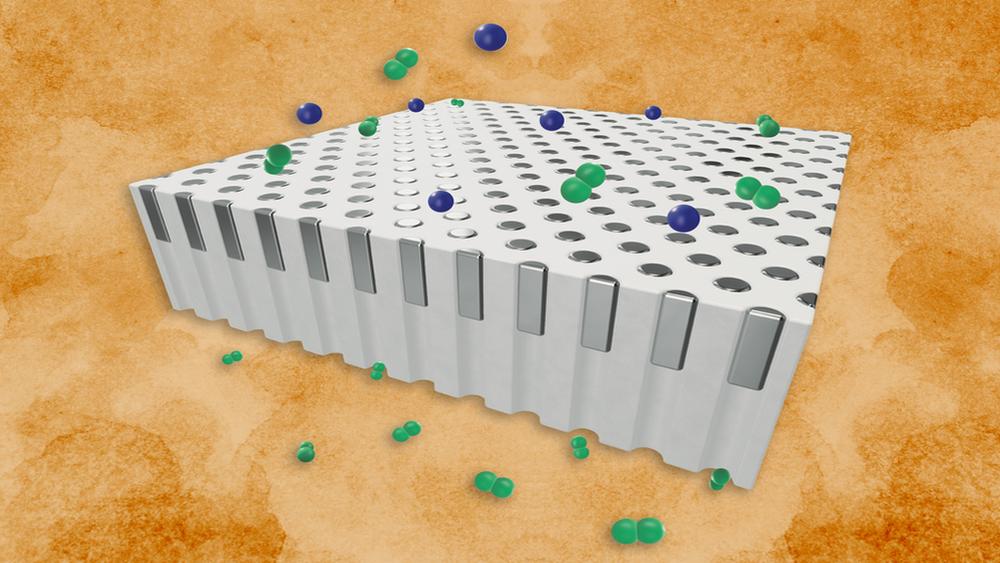
Schematic illustration of a membrane showing selective permeation of hydrogen (green) from a mixture of hydrogen and helium (blue) gases
Palladium is a key to jumpstarting a hydrogen-based energy economy. The silvery metal acts as a natural gatekeeper against every gas except hydrogen, which it readily allows through. For its exceptional selectivity, palladium is considered one of the most effective materials for filtering gas mixtures to produce pure hydrogen.
|
ADVERTISEMENT |
Today, palladium-based membranes are used on a commercial scale to provide pure hydrogen for semiconductor manufacturing, food processing, and fertilizer production, among other applications where the membranes operate at modest temperatures. If palladium membranes get much hotter than 800 kelvins, they can break down.
Now, MIT engineers have developed a new palladium membrane that remains resilient at much higher temperatures. Rather than being made as a continuous film, as most membranes are, the new design is composed of palladium deposited as “plugs” into the pores of an underlying supporting material. At high temperatures, the snug-fitting plugs remain stable and continue separating hydrogen rather than degrading as a surface film would.
…
Add new comment