Kevin Coughlin/Brookhaven National Laboratory
Lakshmy Kannadi Valloli of Brookhaven Lab’s Chemistry Division performing experiments at Brookhaven’s Laser Electron Accelerator Facility. These experiments created a solvent radical and followed the chemistry as it produced a previously unknown intermediate form of a nickel-based catalyst.
Ateam of scientists across several U.S. Department of Energy national laboratories has unraveled how light and a previously unknown form of certain nickel-based catalysts together unlock and preserve reactivity. This research, described in the journal Nature Communications, could potentially advance the use of abundant nickel in place of more expensive palladium in industrial chemistry.
|
ADVERTISEMENT |
The collaborative research effort was spearheaded by Energy’s National Renewable Energy Laboratory (NREL). It involved scientists from Energy’s SLAC National Accelerator Laboratory, Brookhaven National Laboratory, and Argonne National Laboratory.
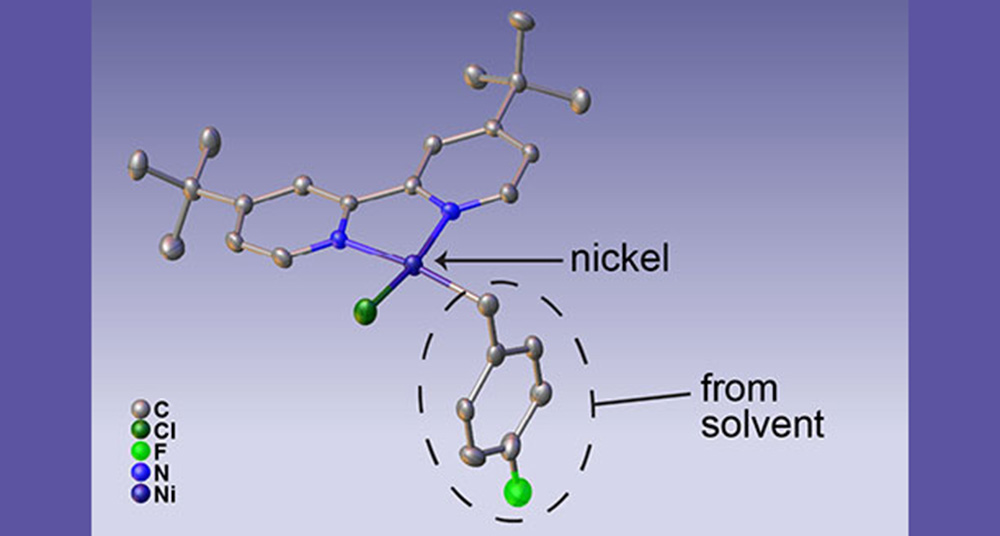
Structure of a previously unknown intermediate form of a nickel catalyst. The nickel atom is highlighted, and the portion of the molecule formed when the solvent radical reacts with the activated nickel is circled. Credit: Max Kudisch, based on X-ray crystal structure determined by him and Rebecca Smaha/NREL.
…

Add new comment