(AssurX: Morgan Hill, CA) -- AssurX, Inc., a leading enterprise quality management, risk, and regulatory compliance solution provider, has released the latest update to its AssurX audit management software.
|
ADVERTISEMENT |
Key benefits to the organization
The AssurX software update keeps all stages of the audit process efficient and ensures important activities and issues are being handled properly and on time. The many features help organizations manage risk while meeting regulatory requirements and compliance.
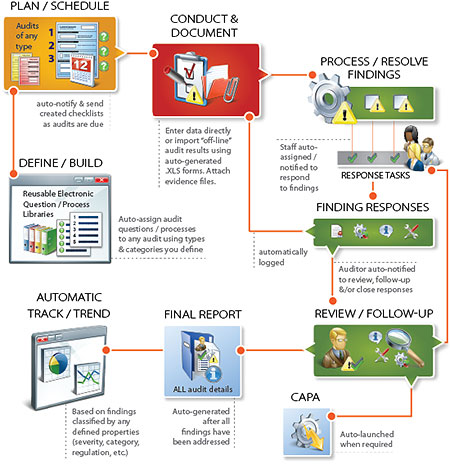
Reduce audit cycle times and increase audit consistency
With the best-practices workflow of this solution, clients reduce errors and missing records. As a result, audit cycle times are significantly reduced.
…
Add new comment