Medical device warning letters and domestic inspections continue to show a slow decline, according to a new report issued by the U.S. Food and Drug Administration (FDA). The number of medical device-related warning letters dipped to 121 during calendar year (CY) 2014, compared to 144 during the prior reporting period.
|
ADVERTISEMENT |
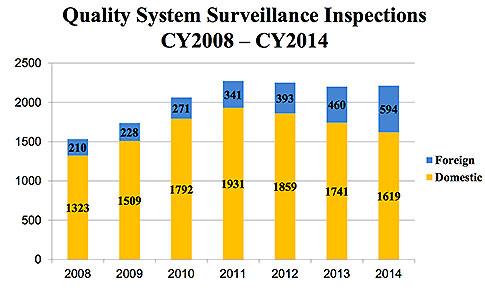
For the fifth year in a row, U.S. inspections declined. They dropped to 1,619 in 2014, compared to 2011’s 1,931, and 2013’s 1,741. However, at least one thing remained constant: Production and process controls, and corrective and preventive actions (CAPA) continue to be the most frequently cited quality management system subsystems.
More than half of CAPA observations were related to 21 CFR 820.100(a) and 21 CFR 820.198(a), and a bit more than 10 percent cited 21 CFR 820.90(a).
…
Add new comment