Body
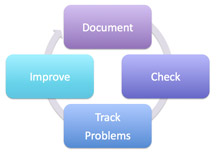 |
| Figure 1: Closed-loop process for managing regulatory compliance |
In Part I, Part II, and Part III of this compliance series, I have described the benefits of using a closed-loop process for managing regulatory compliance (illustrated in figure 1).
…
Want to continue?
Log in or create a FREE account.
By logging in you agree to receive communication from Quality Digest.
Privacy Policy.
Add new comment