There’s new technology that can improve drug quality, address shortages of medicines, lower drug costs, and bring pharmaceutical manufacturing back to the United States. At the U.S. Food and Drug Administration (FDA), we’re focused on propelling these innovations, collectively referred to as advanced manufacturing.
|
ADVERTISEMENT |
Advanced manufacturing, which includes various technologies, such as continuous manufacturing and 3D printing, holds great promise for improving the U.S. market for drugs and biologicals.
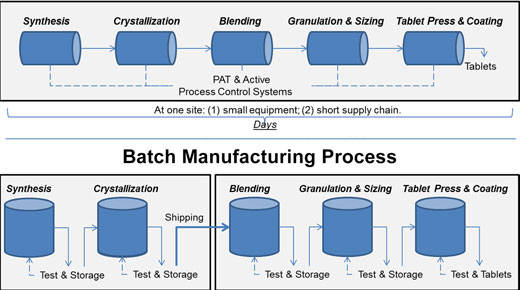
Continuous vs. batch manufacturing
Consider continuous manufacturing. These methods integrate traditional step-wise manufacturing processes into a single system that’s based on modern process monitoring and controls. This enables a steady output of finished drug products even as raw materials are continuously added to the closed system. The closed and continuous nature of these manufacturing systems means that the process is easier to control. These systems also require smaller footprints to operate.
And they’re far more efficient than standard manufacturing processes.
…
Add new comment