(FDA: Silver Spring, MD) -- The U.S. Food and Drug Administration has published an Electronic User Guide for its Accredited Third-Party Certification Program Portal.
|
ADVERTISEMENT |
In this voluntary program, established by the FDA Food Safety Modernization Act, the agency recognizes “accreditation bodies” that may accredit third-party “certification bodies.” The certification bodies will be able to conduct food safety audits and issue certifications for food facilities, for both human and animal foods, and the foods they produce.
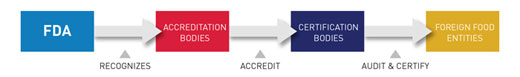
The user guide is designed to help entities apply for recognition as accreditation bodies through the portal. The user guide will also help recognized accreditation bodies manage their accounts, including profiles of certification bodies they have accredited, in the portal.
…
Add new comment