Body
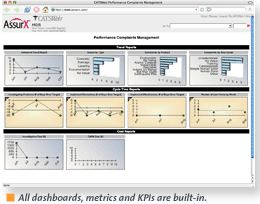 AssurX Inc. recently released CATSWeb Complaints Management & Regulatory Reporting System, its next-generation suite for the medical device manufacturing industry. A comprehensive solution, CATSWeb Complaints Management & Regulatory Reporting features solutions for business-performance evaluation and management dashboards, best-practices workflows, integration with other processes, audit trails and electronic signatures, and reportability-evaluation and regulatory reports.
AssurX Inc. recently released CATSWeb Complaints Management & Regulatory Reporting System, its next-generation suite for the medical device manufacturing industry. A comprehensive solution, CATSWeb Complaints Management & Regulatory Reporting features solutions for business-performance evaluation and management dashboards, best-practices workflows, integration with other processes, audit trails and electronic signatures, and reportability-evaluation and regulatory reports. “We designed the complaint handling and regulatory reporting system in a way to help medical device companies streamline their processes,” says Eric Cooper, AssurX director of business development. “By using the decision-tree methodology, we are providing a system that not only automates the intake events through regulatory reporting processes, but also provides ease of use at all levels of the organization.”
For more information, visit www.assurx.com/managecomplaints.html.
…
Want to continue?
Log in or create a FREE account.
By logging in you agree to receive communication from Quality Digest.
Privacy Policy.
Add new comment