| 
by Peter Unger
The following is an editorial by A2LA president Peter Unger, in part to explain the necessity for mutual recognition agreements among accreditation bodies internationally, and to explain why A2LA withdrew from the National Cooperation for Laboratory Accreditation.
As almost any calibration or testing lab will tell you, getting accredited is an expensive, time-consuming and sometimes confusing process, particularly in the United States, where labs have a choice of accreditation bodies (AB). Do you choose the American Association for Laboratory Accreditation (A2LA), Assured Calibration Laboratory Accreditation Select Services (ACLASS), International Accreditation Service (IAS), Laboratory Accreditation Bureau (L-A-B), National Voluntary Laboratory Accreditation Program (NVLAP) or one of the other dozens of accreditation bodies? If your company is accredited by one of these ABs, will your certificate be accepted by all your customers, either in the United States or in other parts of the world? A recognized AB accreditation can mean the difference between gaining and/or keeping a customer, or losing one. Some labs hedge their bets by getting accredited by multiple ABs and, of course, pay a steep price for the security.
Laboratories are understandably frustrated by the need to obtain duplicate accreditations for similar or identical types of tests, measurements and calibrations. The proliferation of ABs, each with users that accept only their preferred accreditation body, tends to increase the duplication. Examples abound: One company in New York claims it maintains 29 accreditations. This means paying each AB all required fees and receiving audits and/or assessments from each accreditation body. True, some of these assessments are by manufacturers or purchasers of products or services and aren't fee-based accreditations, but either way, the company spends a great amount of time and money managing the visits and staying on top of the fees.
It's generally agreed that the ideal of "one test, one accreditation--accepted everywhere" is a worthwhile and achievable aim. The A2LA supports this aim and believes the way to achieve it is through mutual-recognition arrangements (MRAs) among the accreditation bodies themselves.
Efforts to realize this ideal have a long history, both nationally and internationally. The International Laboratory Accreditation Cooperation (ILAC) was established 30 years ago to develop accreditation as a trade-facilitation tool. To succeed on a global level, accreditation bodies would have to accept the outcomes of each other's accreditations and operate with equivalent criteria and processes. Internationally accepted standards of practice for laboratory accreditation were needed. The first international standard from the International Organization for Standardization, ISO Guide 25:1978, addressed the general requirements for laboratory competence, based upon the work of ILAC. American Society for Testing and Materials (ASTM) standard E548 served as a primary source for Guide 25 text. The latest version of the standard is ISO/IEC 17025:2005.
Beginning in the early 1980s, standards for the operation and acceptance of accreditation bodies were published. ASTM standard E994 was the U.S. equivalent (using virtually identical text) to ISO/IEC Guide 58, which was recently replaced by ISO/IEC 17011:2004. During the 1990s, ASTM committee E36 began to adopt ISO laboratory accreditation standards, which are now the generally accepted U.S. standards. ASTM E36 recommends the formal adoption of ISO/IEC 17011:2004 as a U.S. national standard. The National Institute of Standards and Technology (NIST), the American National Standards Institute (ANSI) and several federal agencies already use these standards.
ILAC also agreed to the rules for peer evaluation of accreditation bodies for MRAs. The foundation for realizing universal acceptance was thereby laid: Acceptance would begin with the accreditation bodies themselves. The whole purpose of an MRA is to provide a mechanism whereby reports from accredited laboratories are accepted everywhere. MRAs, as agreed on regional and international levels, oblige each signatory to recognize and promote the equivalence of other signatories' accreditations. The ILAC MRA was established in October 2000.
ILAC works through recognized regions, or cooperations, so that signatories to the MRAs of the European Cooperation for Accreditation (EA) and the Asia Pacific Laboratory Accreditation Cooperation (APLAC) automatically become eligible for recognition under the ILAC mutual-recognition arrangement. The Inter-American Accreditation Cooperation (IAAC) is in the process of having its MRA process recognized and accepted by ILAC, perhaps as early as November 2006.
To understand how these MRAs work, and their value, you must first understand the structure of the global accreditation recognition system, as seen in figure 1 below. At the top of the structure is ILAC, which oversees, develops and facilitates accreditation guidelines and MRAs. An accreditation body can either sign on as an MRA signatory directly with ILAC or with one of the regional cooperation bodies described earlier. When an accreditation body signs an MRA with either ILAC or one of the regional cooperatives, it's agreeing to accept the certification of any lab that's been accredited by any of the other ABs that are also MRA signatories. So the certificate of a lab accredited by the China National Accreditation Board for Laboratories, a member of APLAC, would be accepted by FINAS in France (an EA signatory) or by A2LA in the United States, a direct ILAC signatory.
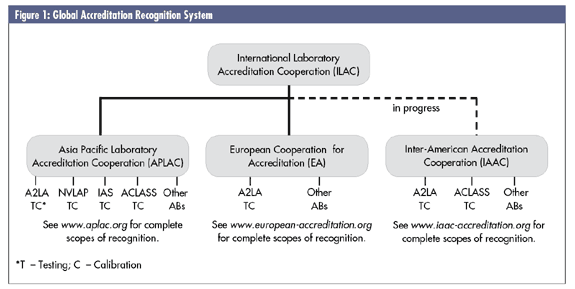
The value for labs is that a lab accredited by, say, IAS, A2LA or NVLAP in the United States would be confident that their certificates would be accepted by an Austrian customer that normally accepts certificates from one of the EA accreditation bodies.
This all sounds good, and it is, but in the United States, the situation hasn't run quite as smoothly.
Laboratory accreditation has developed in many U.S. market sectors at different times and under different circumstances. Dozens of ABs exist in the United States, and all are not created equally--or equally accepted. As accreditations overlapped and became duplicative, ways for consolidating them were explored.
In 1992, the American Council of Independent Laboratories (ACIL), ANSI and NIST formed a tripartite cooperation called the Laboratory Accreditation Working Group (LAWG). After five years of intense discussion about how to reduce the duplication and complexity of the U.S. laboratory accreditation scene, ACIL, ANSI and NIST jointly established the National Cooperation for Laboratory Accreditation (NACLA) in 1997.
NACLA developed a process for evaluating and mutually recognizing accreditation bodies based upon international standards and practice. In October 2000, the first three signatories (A2LA, NVLAP and IAS) to the NACLA mutual recognition arrangement were recognized based on peer evaluations of the APLAC MRA. Later, NACLA added five more signatories to the agreement.
It seemed as though the United States was on its way to an MRA system that would benefit both labs and accreditation bodies. But for NACLA members to have global recognition (other than also signing with existing ILAC members, a duplicative effort that ABs are trying to avoid), NACLA itself would have to become a member of ILAC, like EA or APLAC.
NACLA recently approached ILAC to explore acceptance as a region. However, recognized regions of ILAC must be composed of a minimum of four countries to have international credibility that the decision-making process is impartial. NACLA has argued that the United States is 50 states (or economies) and should be accepted, but it seems unlikely that ILAC is going to bend the rules.
Because of NACLA's unsuccessful bid for ILAC recognition, as well as other reasons, the original three signatories resigned from the NACLA MRA. A2LA continues to support the goals of NACLA and believes that an opportunity to realize them is at hand.
ILAC will likely encourage interested NACLA members to participate in the ILAC mutual recognition process, either directly or through participation in the MRA of one of ILAC's recognized regions (e.g., APLAC or IAAC). This would ensure the maintenance of an international, harmonized process for mutual recognition.
NACLA also intends to pursue closer association with IAAC. A2LA is pleased to support NACLA joining IAAC (or APLAC). The combination of each body's limited resources would help each realize their common goals. U.S.-based accreditation bodies can be evaluated by an internationally recognized process, which enables the accreditation bodies to gain international as well as national recognition with one evaluation. This avoids duplication and the distinct possibility of differing standards of practice. Conflict-of-interest issues would be resolved. Unnecessary trade barriers would be avoided. NACLA stakeholder members can participate in IAAC and ILAC, and thus have more influence on developing international standards of laboratory accreditation practice, which, as noted earlier, are also the national standards.
Just as laboratories don't want duplicative assessments, accreditation bodies don't want duplicative evaluations. Separate evaluation schemes are costly, and if they're based on different standards, they're even more costly. Such costs are inevitably passed through to the accredited laboratories. If an accreditation body can get an evaluation to serve both national and international recognition, costs would be reduced.
Notwithstanding growing discontent in some quarters, globalization is here to stay. This is even more true for accreditation, mutual recognition processes and the standards by which they operate. Global MRA processes are growing in coverage, effectiveness and acceptability. Trade agreements now include references to the ILAC MRA. Recognition and acceptance of the ILAC MRA will continue to grow in the marketplace and with federal agencies. NIST, the U.S. Department of Defense and the Nuclear Regulatory Commission use the ILAC MRA. Other regulators and specifiers will follow, albeit more slowly.
The United States, with its multiplicity of accreditation bodies, isn't unique in the world. At least 10 other countries have more than one accreditation body, many of which are members of ILAC. It's in the interest of U.S. laboratory accreditation bodies and labs to follow the international (and national) rules, the same as other countries. Going our own way would be duplicative, wasteful and ultimately counterproductive.
If U.S. lab customers and accreditation bodies would recognize each other, it would save labs time, money and productivity. We want readers to support and promote "one test, one accreditation --accepted everywhere." It's hoped that, after reading this, readers will gain a better understanding of the ILAC MRA and have faith in the process.
Peter Unger is president of the American Association for Laboratory Accreditation (A2LA), a nonprofit, membership organization administering one of the largest, comprehensive laboratory accreditation systems in the world.
Unger is also vice chair of the International Laboratory Accreditation Cooperation (ILAC). Prior to A2LA, he was associate manager of laboratory accreditation at the National Bureau of Standards (now the National Institute of Standards and Technology). |